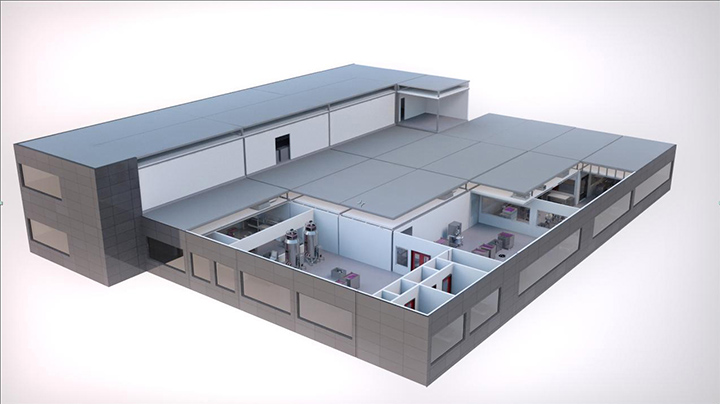
KUBio is a prefabricated, cGMP-compliant facility and process solution for scalable and cost- efficient monoclonal antibody (MAb) production. Pre-validated modular units and processing equipment are delivered to your chosen site where they are assembled, qualified, and ready-to-run within 14 to 18 months. This short timeline is achievable because the KUBio solution includes end- to-end support designed to simplify the process.
Your process equipment
KUBio utilizes the FlexFactory biomanufacturing platform to reduce operational costs through faster deployment, accelerated production turnaround, multiproduct processing, and scalable production. FlexFactory is comprised of single-use technologies and process hardware integrated through automation. It provides flexible manufacturing capacity and strict manufacturing controls that can be replicated for added process quality and consistency.
Your facility
KUBio is a turnkey solution for a rapid response to local healthcare requirements. All KUBio modules are produced in accordance with Chinese, European, and American regulatory standards. The resulting cGMP-compliant facility with optimized footprint design is capable of producing 100 kg of MAbs per year.
Your services
KUBio facilities are supported by a suite of services that provide comprehensive collaborative assistance.
From the start, GE is the single point of contact. GE project managers coordinate the entire process, engaging GE global reach as needed to facilitate importation, regulatory compliance, and in-country assistance. GE can also provide financial assistance through established lending partners.
Our experienced process engineers can optimize your process development and support analytical development. In addition, cGMP pilot batches can be produced at a GE facility while your KUBio is under construction. Your support continues after commissioning with process transfer, training, and repair services provided on location.
While the 62 modules needed for the facility construction of the KUBio are constructed in Germany JHL was preparing the ground and infrastructure at its site in Wuhan, China and the components of the Single use FlexFactory, were manufactured in Westborough, USA, and in Uppsala, Sweden.
Why is building a KUBio facility so much faster?
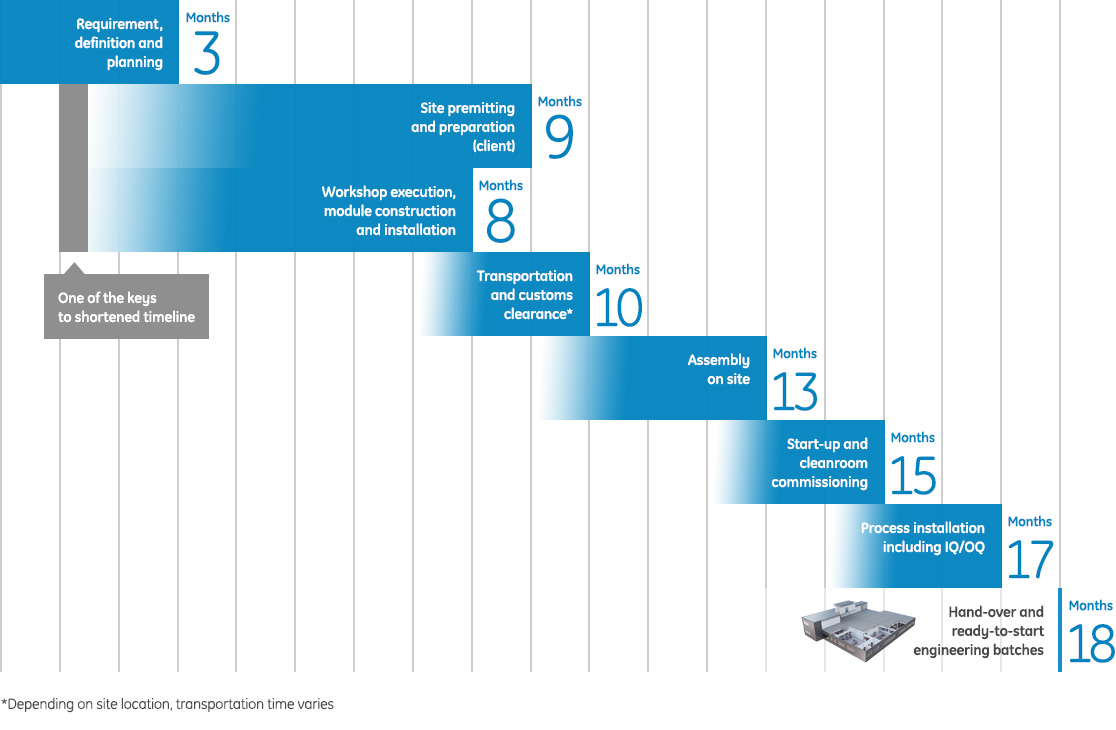
Planning ahead is a critical element for cutting time. Because the conceptual design and most of the basic design is already completed at the time of the investment decision months are gained already at the early start of the project.
More time is gained when the production of the modules is completed while the ground is being prepared at the future facility site.
KUBio project timeline
GE has broad expertise in upstream and downstream processing optimization. In order to meet their precise manufacturing needs, JHL selected ÄKTA chromatography systems, FlexFactory components, and all automation and control parts needed for start-to-finish MAb manufacturing.