Biopharmaceutical manufacturing processes and process development require robust and high productivity solutions to stay competitive. When your business requirements fall outside the standard offerings on the market, our custom services offer joint projects to develop a solution to fit your specific needs.
If the chromatography resin you require is not part of our standard product offering, our specialists can custom design your chromatography resin from an appropriate base matrix and ligand.
For over 20 years, we have provided BioProcess customers worldwide with custom-designed chromatography resins suitable for large-scale and GMP-regulated manufacturing of biopharmaceuticals. Many of our custom resins have later become part of our standard product offering such as Capto Core 400, Capto AVB, and Capto PlasmidSelect.
We work with you to develop and produce custom combinations that fulfill your requirements regarding binding capacity, selectivity, and regulatory support documentation. We give you the flexibility to choose your own ligand, one of our ligands, or a third-party ligand.
For more information about custom chromatography resins please contact your local Cytiva representative.
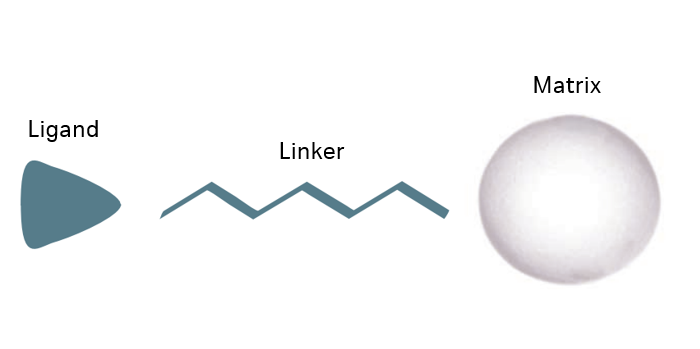
The key components of any chromatography resin are the base matrix, the ligand, and the coupling chemistry. Together, these parameters provide high selectivity, capacity, and pressure/flow properties of the chromatography resin, which enables cost-effective purification.
Our custom specialists have access to the full range of our base matrices, like the Capto and Sepharose Fast Flow ranges. Each base matrix has its own profile in terms of pore and bead size, where the optimal choice can be combined with the relevant ligand for your application and process.
Step 1
- Technical discussion under a CDA1
- Development of small-scale prototypes under an MTA2
- All prototype evaluation performed by the customer
- Mutual evaluation of the potential for a new product for a "stop or go" decision
Step 2
- Evaluation of robustness in the synthesis by Cytiva and performance in the customer process
- Setting a product specification that allows for a robust and high performing production on both sides
- Developing analytic methods for QC testing by Cytiva
- Pilot-scale deliveries from us to you
Step 3
- Scaling up to the required process scale
- Validation of the manufacturing process and developing a data File, MSDS, and Regulatory Support File
- Full-scale deliveries
1 Confidential Disclosure Agreement
2 Material Transfer Agreement
| Product /Project | Product code | Application Area |
|---|---|---|
| Capto AVB | 17372200 | Purification of adeno-associated viruses |
| Capto Core 400 | 17372400 | Purification of viruses and large proteins |
| Capto PlasmidSelect | 17549900 | Purification of supercoiled DNA |
| KappaSelect | 17545800 | Purification of Kappa Fab fragments |
| LambdaFabSelect | 17548200 | Purification of Lambda Fab fragments |
| VIISelect | 17547700 | Purification of Factor VII |
| VIIISelect | 17545000 | Purification of Factor VIII |
| IXSelect | 17371400 | Purification of Factor IX |
| Alpha1AntitrypsinSelect | 17547200 | Purification of Alpha 1 Antitrypsin |
| Capto Blue (high sub) | 17545200 | Purification of misc. pseudo affinity targets |
| Capto Q XP | 17547300 | Purification of large proteins (IgG) |
| Capto DeVirS | 17546600 | Purification of different viruses |
| Capto Lentin Lectin | 17548900 | Purification of different glycol proteins |
| Capto Heparin | 17546200 | Purification of misc. enzymes and proteins |
| Capto Butyl ImpRes | 17371900 | Purification of misc. proteins |
| Capto Phenyl ImpRes | 17548400 | Purification of misc. proteins |