Reduce costs and increase efficiencies by implementing continuous biomanufacturing strategies into your next process. Continuous biomanufacturing is a key step toward promoting drug quality and improving manufacturing productivity leading to lower drug prices.
In February 2019, the FDA released a statement regarding its approach to advancing pharmaceutical manufacturing. FDA Commissioner Scott Gottlieb and CDER Director Janet Woodcock acknowledged that one of the most important tools for modernizing the pharmaceutical industry’s approach to drug development is continuous manufacturing (CM). This alternative to traditional drug processing is a solution the industry has been driving toward for more than a decade. While implementing it requires time and money, CM is a key step toward promoting drug quality and improving manufacturing efficiency, which could ultimately lead to lower drug prices. This has become especially critical in the biomanufacturing industry, where treating rare and complex diseases often comes with a high price tag that many patients struggle to afford.
An enabler for CM is the concept of connected manufacturing, where continuous unit operations are connected not just physically but also digitally using automated solutions. This approach to drug production gives companies the ability to manage and maintain product quality from start to finish using a fully integrated and connected monitoring and controls system.
Interest in CM has grown considerably over the years, and the recent statement from the FDA shows the agency acknowledges it as a viable solution to advancing and modernizing drug manufacturing. Implementing, and even initially considering CM can be a daunting and sometimes overwhelming task; however, breaking it down into the following four steps may provide some much-needed guidance as you prepare your network for the future of biomanufacturing.
1. Set Appropriate Goals For Your Specific Needs
Every company producing pharmaceuticals strives to produce a safe and effective product with the highest level of quality. Yet, while it is easy to identify this as an obvious goal, identifying a path to do so is much more challenging. You must first determine the demand for a product, how much product needs to be made to meet that demand, and the timeline for delivery. Often, assumptions are made to achieve these first steps, impacting subsequent decisions about the most appropriate way to use the available manufacturing network to meet the demand and timeline. These next steps require an in-depth assessment of a wide range of options for process design as well as what manufacturing capabilities are available in a network, both internal and external. If any gaps in capability and capacity are identified, decisions must be made about how to do more with what is available and supplement the network in an efficient manner. Over time, reconsideration, and in some cases, redirection of strategy may be necessary.
Nevertheless, connected/continuous technologies and approaches exist in varying degrees of GMP readiness and can be combined in many ways to meet your company’s drug product demand. These combinations can be implemented in alignment with existing manufacturing networks, while staying within the limits of your organization’s appetite for tackling risks associated with changes to established manufacturing processes. Therefore, decision-making processes begin with awareness of your manufacturing network, staff capabilities, and organizational tolerance for risk and reward in context of both short- and long-term manufacturing strategy.
Beyond the early decisions related to quality and demand, the decision map increases in diversity and complexity with each additional decision point, especially if you are still struggling with articulating your initial needs. This can be overwhelming, especially in the early phases when every decision seems crucial. Therefore, working with a knowledgeable partner with proven technical expertise can guide you through the decisions. They will seek to understand the state of your current development activities and technical capabilities and identify any unknowns to look for opportunities for improvement. Questions they may ask include:
- What is your manufacturing capacity?
- Where can you modernize and intensify to optimize?
- Do you plan to stay in-house or work with a CMO? If the latter, have you selected one?
- What other products are being produced?
- Is there pressure to hit a certain timeline?
The answers to these can help determine where there is room for flexibility and where discrepancies may exist in a current plan. One mistake at this stage is continuing debate around the benefits of connected and continuous manufacturing. The statement from the FDA and progress around the industry, such as Amgen’s next-generation facility in Singapore, are proof this is the future of manufacturing. Debating theoretical points about its validity puts you at a disadvantage to your competitors. And unless you have an unstable molecule or one with other unique attributes that require different unit operations, it is always possible to revisit batch and hybrid processing if continuous is not a fit.
2. Understand The Relationship Between The Decisions You Make
The diversity of today’s biomanufacturing landscape has created a variety of challenges in drug development. Understanding the relationship between the decisions you make gives you the foresight necessary to anticipate future challenges and prepare for them before they arise. For example, there is significant interest across the industry to reduce overall facility footprints. This drives the use of smaller, functionally closed equipment in less classified space. There must also be consideration about how that facility can be built now in a way that is flexible enough for future processes later while also pursuing minimum footprint and reduced capital costs.
If it is a multiproduct or multimodal facility, you must develop and adopt an equipment platform that covers a wide operating range. An efficient workforce should balance the right skillsets to run the facility efficiently and get the most throughput out of your consumables and process materials. In these situations, it may be beneficial to work with a CMO, as they encounter a wide range of customer needs and are familiar with the considerations that need to be made. Another option to defer capital investments until more is known about the likelihood of a molecule’s success is using a modular facility, such as Cytiva’s KUBio. These prefabricated biomanufacturing facilities offer a configurable production line that can substantially reduce costs and setup time, taking your product to market more quickly and efficiently.
Finally, as companies continue to expand globally, other challenges and questions can arise, such as having reliable automation and digital tools to allow a global view of your process. Supplying global facilities with raw materials may be different if the materials originate in the U.S. or Europe versus somewhere else in the world. Coordination requires a reliable supply chain for not just your facility and process, but also within the entire company and your suppliers.
3. Implement Technologies That Drive Better Process Performance
Many of the approved products on today’s market are manufactured using legacy processes. Despite the shift to process intensification, there is often an appeal to stick with approaches that still produce safe, high-quality products. However, process intensification is usually a prerequisite to CM. New, innovative technologies that intensify your process can increase titer and help manage higher volumes of media and buffers, essentially getting more product out of your processes and manufacturing network.
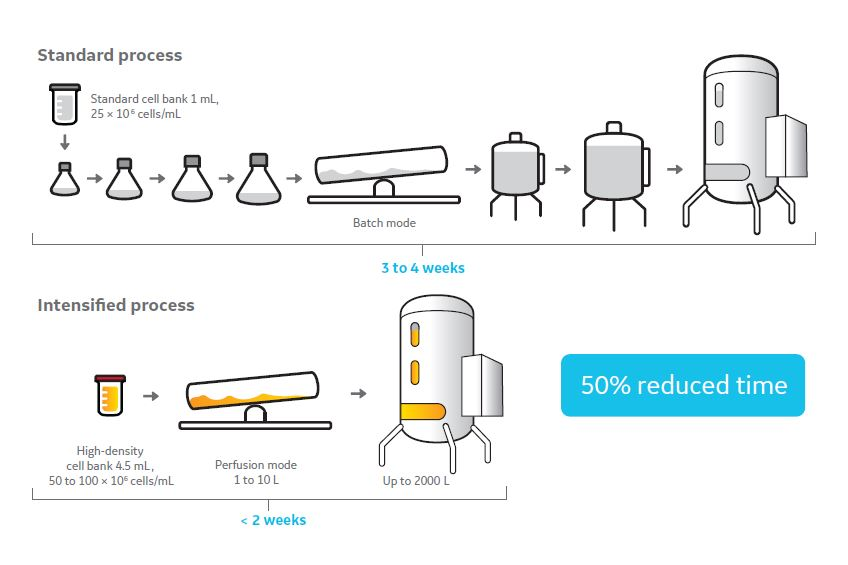
Examples for intensifying upstream processing include implementing high-density cell banks and perfusion in an N-1 bioreactor, which can reduce expansion time by as much as 50 percent. These upstream technologies should balance media and single-use costs, while considering a reduction in time in the early expansion stages could also have a major impact on a manufacturing network and/or how many lots or products could be added. In addition, the application of smaller bioreactors using single-use technology (SUT) could also provide flexibility. With fewer limitations on changeover time and cleaning verification, it becomes possible to run more lots and/or products in a facility.
Fig 1. High-density cell banks and perfusion in an N-1 bioreactor can reduce expansion time by as much as 50 percent, but these upstream technologies should balance media and single-use costs.
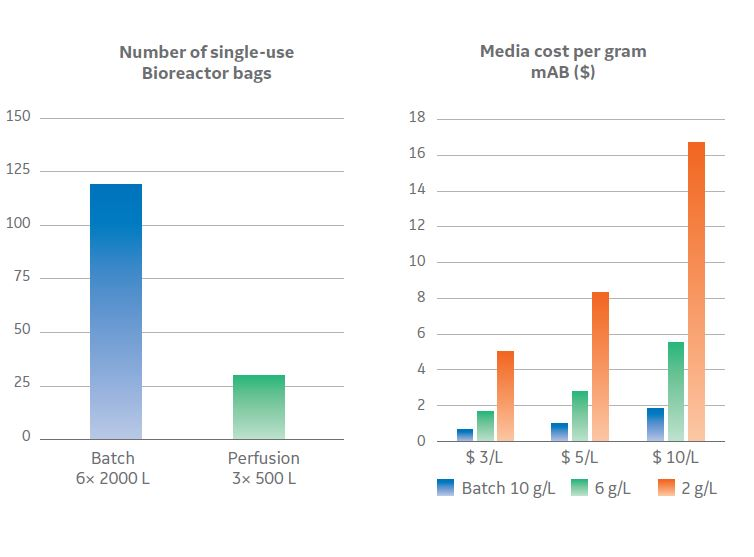
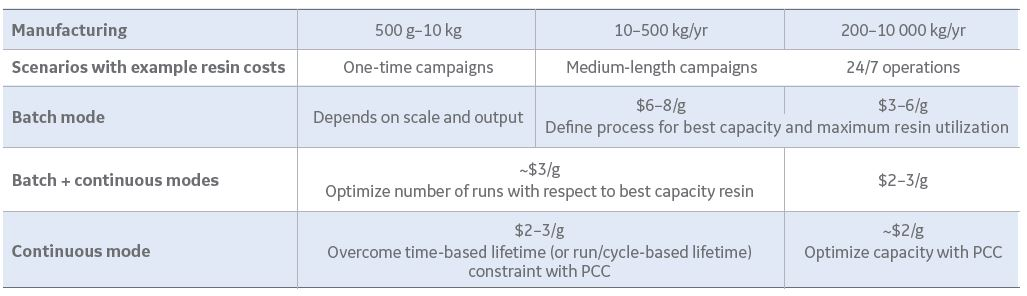
There are so many process design options in downstream, such as resin selection, equipment operating spaces, whether to pack or use pre-packed columns, and other related decisions. An obvious target when using more expensive chromatography resins, such as Protein A, is to get the most value out of them. The approach for batch Protein A chromatography is traditionally based on the best capacity and resin utilization. Interest is turning to continuous chromatography to intensify capacity and utilization beyond current batch levels. Selecting the right operating parameters can be complex since there is a trade-off between capacity and utilization, which becomes more dependent on scale and demand. Despite its complexity, employing a continuous chromatography process can save two to four times the resin cost from a batch process.
Fig 2. Operating parameters can be complex since there is a trade-off between capacity and utilization, are dependent on scale and demand.
Each of these examples, though, is specific to a unit operation, but it is also important to understand the how they impact one another. Therefore, decisions should not be based on only the process science for one unit operation but how each change affects the entire process and manufacturing network. If you set goals and understand the decision relationships, executing the right technology should be straightforward.
When it comes to adopting a connected, continuous system, automation and reducing the time and cost associated with its development become important. It is also crucial to understand any data you generate so you can use it to improve your process. Implementing an effective automation and digital platform that meets your capabilities and needs is key to successful performance. The industry-wide goal is to become more simplified by investing in automated solutions. Taking time up front to select and invest in the right automation and digital solutions allows complex applications to be simplified within the upstream and downstream processes, paving the way for future adoption of more connected and continuous processes.
4. Effectively Execute On Your Strategy
Once you understand your goals, the opportunities available to you, and the impact of each decision you make, you will have a clearer picture of your strategy to meet those goals. Nevertheless, as you execute on a well-planned strategy, you may still encounter unexpected challenges. The nature of batch processing allows you the time to stop production if an issue arises and consider next steps. With continuous, though, these are quick-time decisions that often impact more than one unit operation, and you must be able to justify and defend your decisions in the face of regulatory scrutiny.
Fig 3. Operational examples
The best way to prepare for the unexpected is by leveraging tools designed to optimize your data analysis, achieve operational excellence, and incorporate insights into planning. Planning activities like risk assessments helps determine the possible risks, how you could mitigate them, and develop a contingency plan to address them should they arise. Sometimes, risk assessments are conducted only for filing purposes. Instead, they should be treated as a living document that is referred to and adjusted as needed. Successful execution also includes working with cross-functional teams and SMEs for connected and continuous operations. By breaking down the silos that historically exist in pharmaceutical organizations, you can develop an effective strategy that delivers on the goals identified.
The FDA’s recommendation to implement continuous unit operations is evidence biopharmaceutical manufacturing is moving toward a future that embraces adoption of innovative technologies that can reduce product failure, increase quality, and drive improved efficiency. This is also in line with trends in manufacturing to reduce manual operations and intensify processes to ensure effective utilization of facilities and equipment. Your experiences will develop your base of knowledge for this new era of manufacturing. However, the most effective development of this technology and its use will come from a collective understanding that can be shared across industry. Through this type of collaboration, the implementation of CM can be shared and supported, paving the way for safe, effective, and affordable medication for patients around the world.
Discover more in the Process Efficiency series:
- Optimizing process efficiency in upstream manufacturing
- Achieving operational efficiency in today’s fragmented market
- Intensified chromatography strategies
- How to adapt biomanufacturing processes
- Process Intensification