Optimizing protein transfer is essential to obtain great results in Western blotting. Below, we’ve compiled our top five troubleshooting tips on protein transfer to help you to improve your molecule transfer efficiency.
Sometimes, even though you followed all the protocols and set up everything right, you still encounter problems in blotting your protein onto a membrane. Here are our top five troubleshooting tips on protein transfer to help you to improve your molecule transfer efficiency.
What is transfer in western blotting?
There are many different approaches to Western blot transfer, however generally, is it a term used to define the transfer of biological molecules, such as protein molecules, from the gel to a solid support membrane, which is usually made of a chemically inert substance, such as nitrocellulose or Polyvinylidene difluoride (also known as PVDF). This step makes it possible to then detect the proteins on the membrane using specific antibodies. For more information, read our blog on the Seven Steps to Western Blotting or if you’re a beginner, view our Western Blotting for Beginners video.
5 Western Blot Transfer Troubleshooting Tips:
Sometimes, even though you followed all the protocols and set up everything right, you still encounter problems in blotting your protein molecules onto a membrane.Here are five common issues that researchers and scientists have encountered in protein transfer whilst performing their western blots — and also the solutions on how to deal with them!
1. Western Blot Transfer Troubleshooting: No bands transferred to the membrane
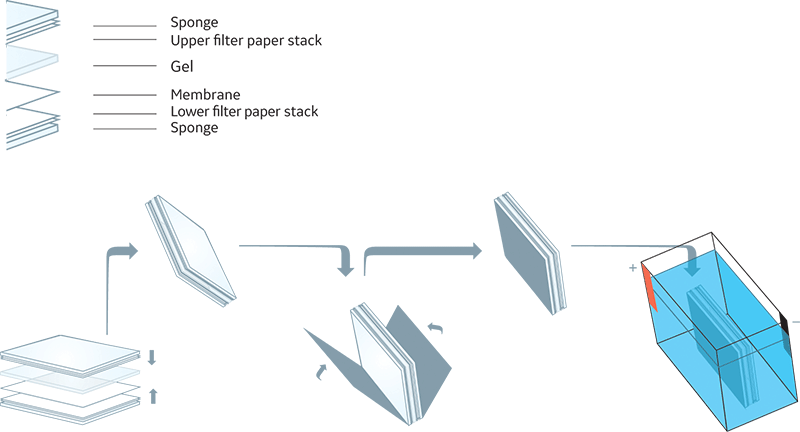
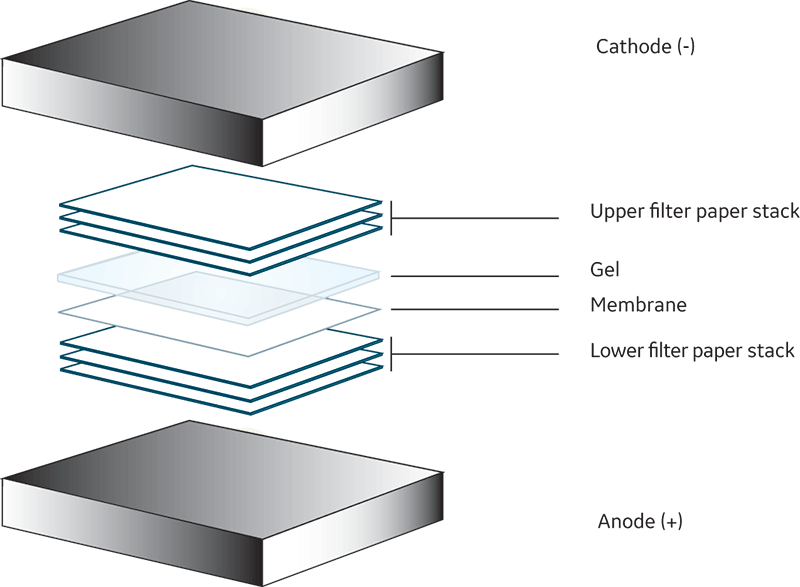
When none of the protein bands appear on the membrane, the most likely reason is problems relating to either the equipment or the assembly of the gel membrane sandwich. The polyvinylidene fluoride (PVDF) or nitrocellulose (NC) membrane should always be oriented on the anode (+) side of the gel. This applies whether the transfer is a wet transfer or semidry transfer (Fig 1 and 2).
Problems with the power supply can make the current too low or run in the wrong direction, all preventing protein transfer and inability to measure molecular markers. To be on the safe side, membranes can be placed on either side of the gel in case the transfer stack has been set up incorrectly or the power supply is incorrectly connected.
Another reason for the absence of protein is a short transfer time. If the transfer setup is assembled correctly, increasing the transfer time might improve protein molecule transfer. Transfer time can be optimized by monitoring the degree of transfer of molecular weight markers included in the Western blot electrophoresis step.
Fig 1. Typical wet transfer setup.
Fig 2. Typical semidry transfer setup.
2. Western Blot Transfer Troubleshooting: Individual bands or entire sections of the blot missing
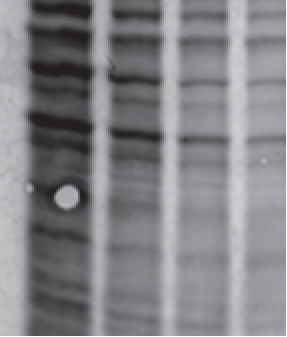
Poor connection between the gel and membrane is a common cause for localized areas with no protein molecule transfer. Ensure air bubbles between gel and membrane are not present as this this could be another possible reason why small areas of the membrane are empty (Fig 3).
An easy trick to remove air bubbles and to avoid loose membranes, which can also reduce transfer efficiency, is to use a pipette as a makeshift rolling pin. Gently rolling the pipette across the membrane can help solve two problems at once for reliable transfer without blind spots.
3. Western Blot Transfer Troubleshooting: Poor transfer of large proteins
Sometimes, a Western blot transfer works well for the smaller protein weights in your sample, but not for the larger sized proteins. Larger proteins sometimes need a bit more encouragement to move to the membrane.The simplest way to get all your protein molecules off the gel is to increase the current or the transfer time, though care is required as these changes can cause issues with overheating and with efficient transfer of the smaller protein weights in your sample (see below).
A second option is to optimize the blotting transfer buffer to improve solubility. Including a small amount of sodium dodecyl sulfate (SDS) in your transfer buffer and using less methanol makes larger protein molecules more soluble and improves migration. For a more thorough understanding, read our blog on choosing molecular markers here.
4. Western Blot Transfer Troubleshooting: Poor bands of small proteins
What about the reverse situation? Clear bands for large proteins, but weak bands for low molecular weight proteins?
A common reason for low signal intensities is a phenomenon known as “blow-through”. This occurs when low molecular weight proteins quickly transfer to the membrane, but pass right through without binding.
Using a membrane with a pore size of 0.2 µm or a membrane made of PVDF, which has higher binding capacity for small proteins, might alleviate the problem. It is also possible to use two membranes on top of each other to avoid the risk of proteins passing all the way through without binding.
Another way to prevent blow-through is to reduce the current or the transfer time for your Western blots.
Traces of sodium dodecyl sulfate (SDS) can interfere with binding of all proteins, but small proteins are even more sensitive; eliminate the problem by equilibrating the gel in transfer buffer containing 15% to 20% methanol for at least 15 min. Do not add sodium dodecyl sulfate to the transfer buffer unless absolutely necessary.
5. Western Blot Transfer Troubleshooting: The gel appears distorted
Gel distortion can result from overheating caused by excessive current flowing through the gel. If possible, try lowering the current. This will protect your gel as well as your protein molecules.
If your protein(s) require(s) a high current, your transfer setup needs to be kept as cool as possible. If you are performing semidry transfer, consider switching to wet transfer and place the setup at 4°C using prechilled transfer buffer.These are some of the common problems we have encountered. We hope these solutions are helpful to you. For more information on carrying out a reliable, clean transfer, download the Western blotting handbook.