Manual buffer preparation in biomanufacturing is ripe for improvement, and inline conditioning can save money, time, and space while ensuring buffer quality.
Manual buffer preparation, although not a complicated activity, is an often-overlooked area for improvement by the biopharma industry even though 24 percent of biomanufacturers state that it causes “severe” or “significantly severe” capacity constraints (1).
The traditional way of making buffers, where buffer components are dissolved in water-for-injection (WFI) in large tanks before transferred into bags and then transported to the point of use, requires significant time and space.
In contrast, inline conditioning (IC) allows for buffers to be produced from single-component stock concentrates when needed, at the point-of-use (POU). IC automates buffer production, saving money, time, and space.
It is important to monitor buffer quality, as it is essential for the success of the entire biomanufacturing process. Naturally, buffers prepared with IC systems must have the same quality as buffers produced manually.
The importance of a buffer release strategy for IC
Buffer production often becomes a limiting factor when the goal is to design more flexible facilities. To overcome this, Janssen Biologics BV decided to investigate IC technology as a buffer preparation alternative, but they also wanted to better understand IC and its impact on the buffer quality.
To support this inquiry, Karolina Busson et al, compared buffers produced with IC and buffers produced manually to see if their quality was equivalent. The study used two different control modes of IC, ratio control with flow feedback and pH/conductivity feedback (Figure 1). A robustness study was carried out to find the needed parameters for error propagation analysis.
Fig 1. Inline conditioning systems can have three different control modes that can be tailored according to your buffer acceptance criteria: flow, pH-Flow, and pH-conductivity. Karolina Busson and her team used flow and pH-conductivity feedbacks.
The study also established a recommended control and release strategy for IC. Any facility relying on IC should implement a buffer release strategy that guarantees the quality of buffers. It is important to understand how the incoming parameters, such as expected recipe precision, stock solutions, temperature, and their variability affect the buffer quality. This helps determine the most suitable control strategy.
Measuring variability in stock solution concentrates
To compare the quality between manually prepared buffers and IC, buffers currently being used for protein purification were formulated and analysed. To find the recommended control and release strategy, Karolina and her team measured how variabilities in stock solution concentrations affected pH and conductivity.
The supporting robustness study deliberately prepared the stock solutions at ±10% from the target concentration. If there was a linear response, the results could be used to predict the degree of error with a smaller deviation.
This was done for pH and conductivity for both control modes. For flow feedback, two different scenarios were simulated: minimum, with no error in the recipe and at maximum with a small error in the recipe.
Conclusion
The results confirmed that buffers produced with the IC technology and traditional way have consistent, and equivalent quality and performance.
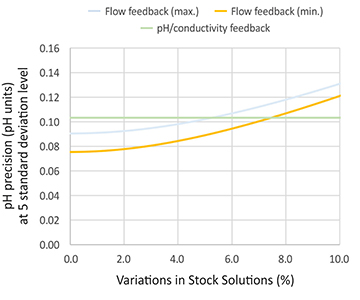
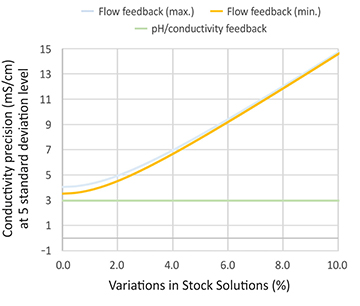
Fig 2. Variation in stock solution for the two control modes. The pH/conductivity feedback is not impacted by the stock solution variation, whereas the flow feedback is more sensitive towards the variation. The graphs are published with the permission of Bioprocessing Journal.
When it comes to choosing the control mode, in situations where the stock solutions are accurate (the uncertainty is below two percent) and the temperature is controlled, the two control modes (flow feedback and pH/conductivity feedback) have the same performance (Figure 2). If there are higher variabilities in stock solution concentrations or process temperatures, pH/ conductivity feedback might be a better option.
Fig 3. Buffers prepared using the inline conditioning technology and the traditional way have equivalent and consistent quality study shows.
The performance of the two control modes, in general, is equivalent to the established procedures while maintaining excellent control over the process parameter precision.
References:
(1) 17th Annual Biomanufacturing Report Bioplan 2020
More information
Free e-learning: Get confident: inline conditioning control modes
Learn more about large-scale bioprocess buffer management