New modalities, like bispecific antibodies, present unique challenges compared to mAb production. For purification, there’s not just one right answer to which strategy to use. Here are some industry insights to help you navigate the bispecific jungle.
The biopharma pipeline is changing. As we move from a mAb-focused industry, new challenges arise with the increased diversification of therapeutic molecules. One of these new types of molecules are bispecific antibodies (BsAbs). This group has several advantages over mAbs. But it also presents unique process development issues to solve.
Bispecific antibodies interact with two different surface antigens. This dual specificity enables a wide range of applications, like blocking two different signaling pathways simultaneously. Today there are three commercially approved therapeutic BsAbs, about 80 in clinical trials, and many more in the preclinical phase.
The increasing number of antibody variants in the biopharma pipeline is striking. We found the same trend in a poll performed at a recent antibody development meeting; the antibody manufacturers had a significant proportion of antibody variants, including BsAbs, in their clinical pipelines.
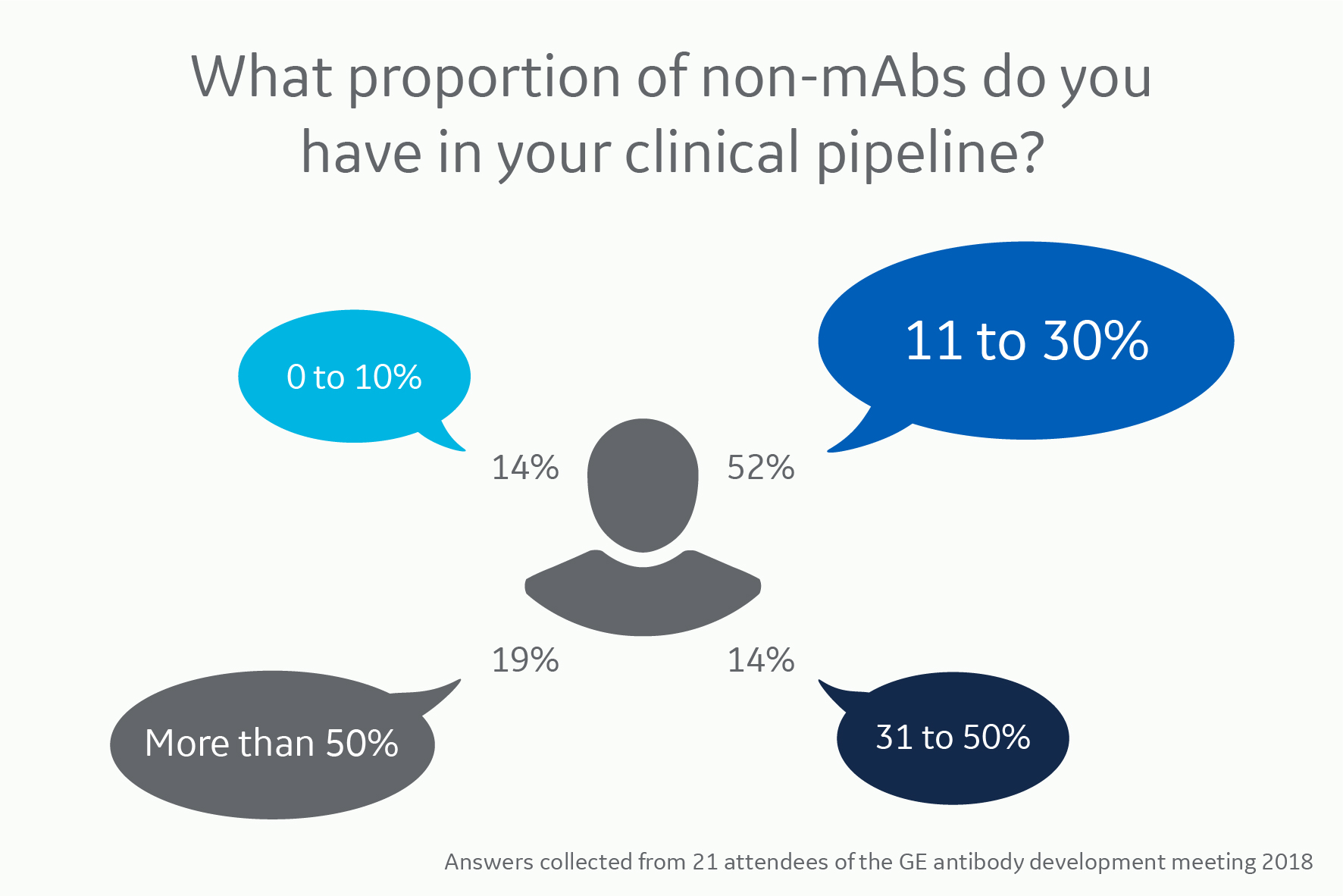
Many molecule variants with as many purification strategy answers
When it comes to purification, the common structure that monoclonal antibodies share, means manufacturers can use a platform approach. Usually, this boils down to using protein A chromatography for capture of the antibody. But for purification of BsAbs, the solution is not always as straightforward.
It’s a diverse group of molecules, so different bispecific antibodies need different purification solutions. There’s not just one correct answer to which purification strategy to take. Instead you need to base your decision on which principle to use on the property of the impurities and target molecule.
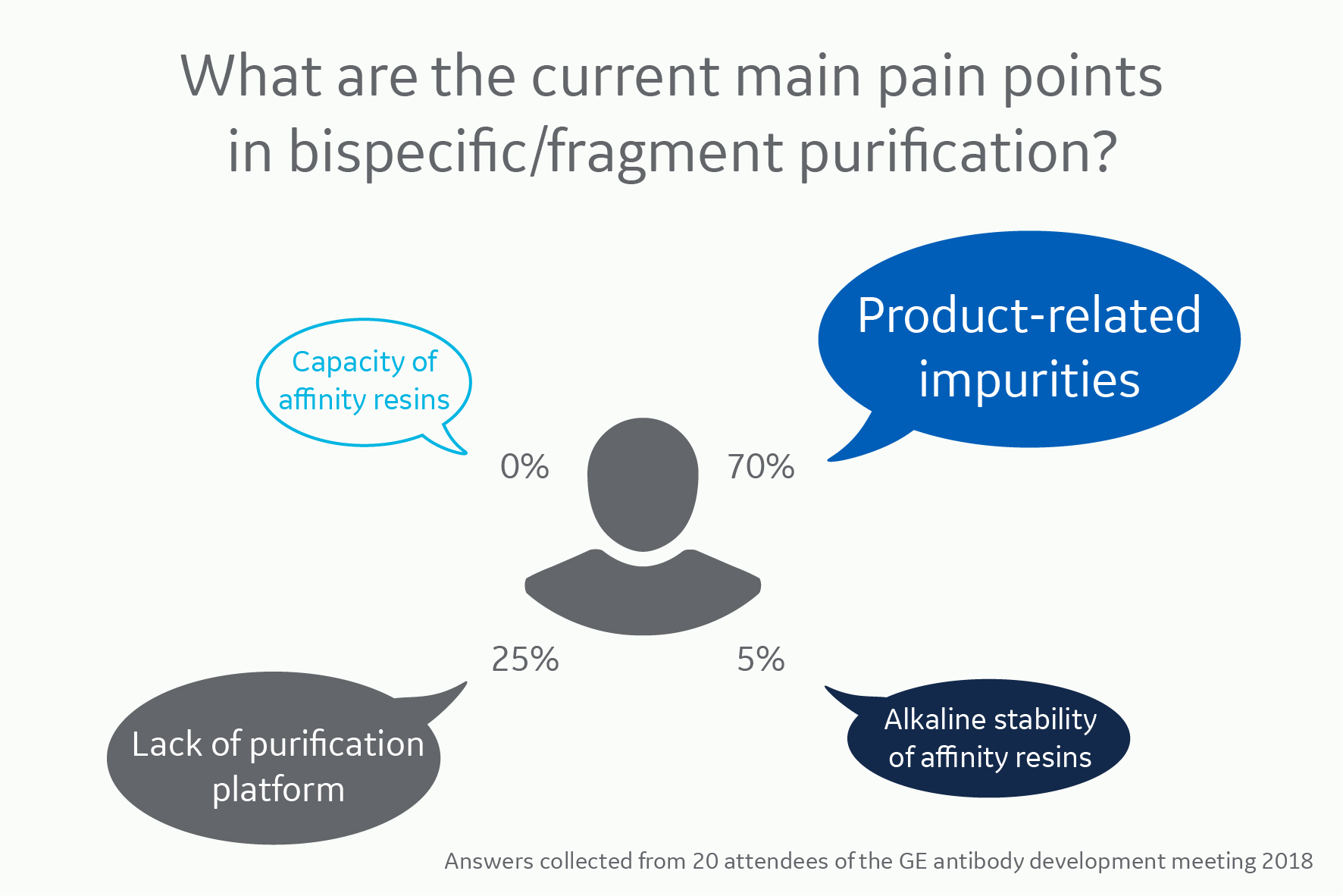
Main pain points in bispecific antibody purification
It’s obvious that purifying bispecifics presents new hurdles for process developers. Apart from the lack of purification platforms, product-related impurities present a much larger problem for BsAbs than conventional mAb processing.
The reason for this is that multiple variants of the antibody are formed during the expression. For example, you get homo- instead of heterodimerization of heavy chains. You also get random binding of light chains to heavy chains. This results in lower yields compared to mAb production.
Dr. Andrew Tustian, Associate Director at Regeneron in the US, has seen the rise of new modalities over the last five years or so. He co-leads the Purification Development Group within Preclinical Manufacturing and Process Development and agrees that one of the main pain points is the increased level of impurities.
“Everybody has their own additional impurities from bispecifics to deal with,” Tustian says. “It can be mismatched antibody chains, misfoldings, and so on. And the impurities are often very similar to your target. That makes separating them so much harder than for a conventional purification.”
This challenge was also rated as the main pain point by the majority in our poll. The lack of purification platforms was also ranked high.
Bispecific antibodies are also in focus at UCB in the UK and Belgium. Gavin Wild is Associate Director in downstream process development there. During his 14 years at UCB he’s been part of the shift towards new modalities.
“Production of bispecifics often results in lower total yields and less standardized purification protocols,” explains Wild. “It can be hard to handle expectations from internal stakeholders that are used to more mature monoclonal production.”
The challenge of meeting management expectations was echoed in an expert panel discussion held at the antibody development meeting. In addition, the industry faces growing activities of intellectual properties in molecular design. Over the last 15 years, manufacturers have developed more than 60 different technological platforms for BsAbs.
Case studies: approaching BsAbs purification from two different angles
Two recent collaborations exemplify how to approach purification of BsAbs in different ways. In the first, Regeneron needed a resin to develop a platform for bispecific antibodies. They asked us to develop a new high-resolution protein A resin with affinity for the Fc-region, but no affinity for VH3 region.
In the second, UCB had developed a non-Fc-based molecular platform and needed to address purification for their relatively high diversity of candidate molecules. For this purpose, they explored both non-affinity as well as protein A resins.
Regeneron: designing the target molecule for a platform approach
The possibility to use a platform approach was an important consideration for Regeneron when they designed the target antibody. With a platform approach, they would save time on process development and speed the time to clinic.
“The tradeoff we did was lower titers,“ says Tustian. “We would only reach a maximum of 50% of what we would have in a mAb process. But the speed it gave us was worth it”, he adds.
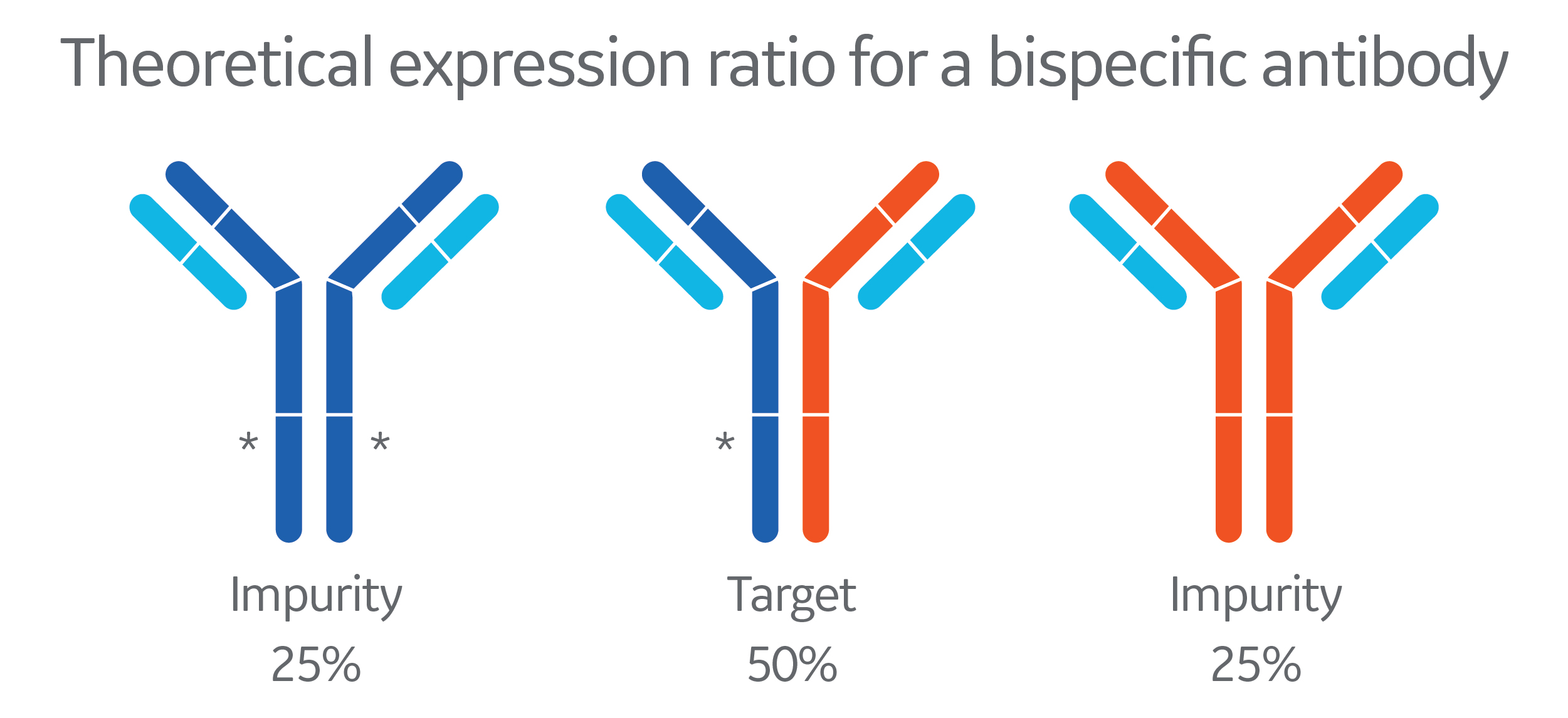
By introducing a substitution in the protein A-binding Fc-region of one of the heavy chains (indicated by a star in the illustration), the molecule design worked well for a platform purification strategy. That way Regeneron quickly had a platform available for BsAbs. The collaboration project also led to the development of a new protein A resin. The chromatography resin minimized unwanted VH3 domain binding but offered the necessary resolution to separate the different antibody variants. (1)
UCB: using a toolbox approach for bispecific purification
UCB chose a very different approach to molecular design and purification strategy. As opposed to Regeneron, they had developed a bispecific, non-Fc-based molecular platform. The diversity of the candidate molecules was relatively high. So, UCB needed to find an effective purification strategy based on a toolbox approach, rather than a platform.
Together with GE’s Fast Trak team, UCB evaluated both non-affinity and affinity resins. The protein A resins had to have a retained affinity for the VH3 region, as this was present in different variations in the molecules.
“Because of the non-Fc-based molecule design, protein A purification was not first choice for us,” says Wild. “We went through many different affinity ligands and polishing solutions. In the end, a protein A resin turned out to be the optimal, most cost-efficient purification solution for us."
Where should bispecific development go from here?
The case studies illustrate that the choice of purification strategy for BsAbs depend on the molecular design. As the number of these molecules grow in the biopharma pipeline, we should view the production of them as a whole—from molecule design, through process development, to manufacturing.
“We need to come at it from both ends and start looking at it as a whole process, including upstream and downstream supported by process-analytics for fast and effective monitoring of the target product, as well as high-resolution methods necessary for control and understanding of the product-related impurities,” says Wild. “Early collaboration, with Discovery during molecule design and between upstream and downstream during process development, will help find the right balance for the entire process.”
The diversification of the pipeline creates new challenges in downstream processing. Rather than a single purification platform, we’re likely to see toolboxes for different design variants. The industry will also need flexible purification solutions when batch sizes go down and number of projects increase.
“To solve these new challenges, manufacturers and suppliers need to work closer in future,” says Tustian. “If suppliers get involved early on, we can work together to find solutions quicker.”
If you’re interested in purification of BsAbs, check out this article on development of a protein A chromatography process for bispecific antibodies.