Control strategies that assure product quality and process performance are essential in biomanufacturing. Here are the pros and cons of five control strategies that can provide process consistency even in the presence of chromatography resin variability.
In biomanufacturing, process robustness is critical to limit unexpected variations in product quality and process performance. Developing a solid control strategy is one way to ensure process robustness. The strategy itself should be defined from deep process understanding and process characterization.
For a chromatography step, the control strategy should mitigate the impact of process variability on critical quality attributes (CQAs). This can arise from variability in process parameters as well as raw materials or feed materials. Most chromatographic steps are inherently robust to variability in resin attributes such as ligand density or particle size, but variability can occur for challenging purifications, for example for new modalities.
In this post, you’ll find five control strategies to prevent process variation due to resin variability. These strategies can be used when the interplay of process parameters and resin have been identified to potentially impact process outcome during process characterization work.
- Shifting process parameter values
- Adaptive control strategy
- Lot mixing
- Custom specifications
- Lot selection
1. Shifting process parameter target values
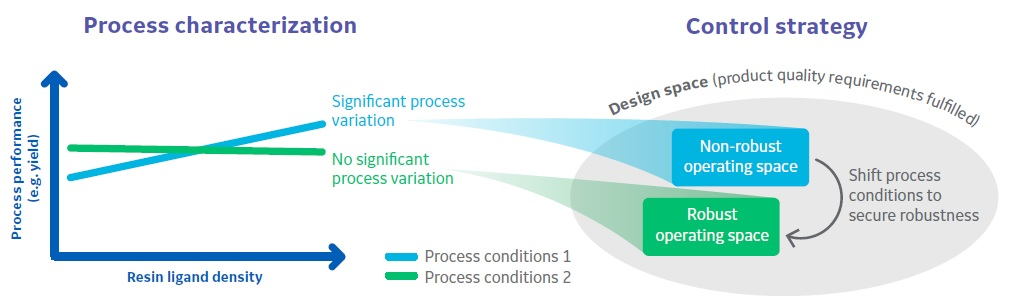
In this option, you shift the settings for one or more process parameters to a process operating point less sensitive to resin variability (Fig 1). Examples include pH and conductivity set points or reduced/increased load ratio.
The possibility of shifting process parameter target values and/or ranges to account for variability is a simple and robust strategy.
Fig 1. Shifting process parameter target values is a simple and robust control strategy. For chromatography resins, study how the interplay of process parameters and resin variability impacts process outcome during process characterization. Then define process parameter target values that minimize the impact of resin variability.
2. Adaptive control strategy
With this option, you avoid always having to perform under worst-case scenario conditions. Instead, the adaptive control strategy allows the settings for process parameters to be adjusted to the properties of a given chromatography resin lot.
That way you can get maximum process performance for the entire resin variability range, while still ensuring consistent product quality. Examples of adaptive processes are:
- Alter set point of wash length based on a function test for each resin column pack.
- Adapt load ratio to the properties of the feed material and resin attributes.
Using an adaptive control strategy for resin variability is an approach that provides an improved overall process economy and robustness. Manufacturing operation teams might, however, consider this approach more complex than others.
3. Lot mixing
With this option you reduce the overall variability by mixing resin lots already present in your inventory. The average value of any resin attribute in the mixture will, of course, tend towards the middle of the specification. But that doesn’t necessarily guarantee that the performance of the mixture is the same as what the average indicates.
The lot mixing strategy is often not sustainable in the long run as it relies on you having different resin lots with different resin attributes in stock.
4. Custom specifications
If there is no other way to secure the process performance or product quality across the entire resin specification, you can consider a custom company-specific specification.
With this strategy you will have a custom-made resin, with a new article number, that can help maximize process performance and product quality. However, this approach requires a close collaboration with your resin manufacturer and could come at a higher cost than an off-the-shelf resin.
5. Lot selection
In my experience, the lot selection approach is the least desirable control strategy of the ones I’ve listed here. This approach means that you only purchase resin lots that fulfil specific criteria.
The criteria could be based on a narrower specification range that is specific to your company. It can also be based on the outcome of a process-specific use test. But, as the outcome of a use test is unknown beforehand, it’s not possible to ensure a reliable supply of the selected resin lot.
The standard specifications for a raw material, such as a chromatography resin, are there for a reason—to ensure uninterrupted supply to the user. Any attempt to reduce the specification range will therefore put the supply continuity in jeopardy.
Pros and cons of the control strategy options
Of course, no biologic or process is the same and the choice of control strategy depends on your specific situation. For easy guidance, here’s a summary of the advantages and disadvantages of the control strategy options discussed.
| Control strategy option | + | - |
| 1. Shifting process parameter values | Simple to implement | Extended process development work Not always possible to implement |
| 2. Adaptive control strategy | Maximizes process performance and product quality | Might be seen complex by manufacturing operation teams Extended process development work required |
| 3. Lot mixing | Simple to implement | No guarantee that the performance of the mixture is the same as the average indicates. Driving inventory cost and complexity |
| 4. Custom specifications | Maximizes process performance and product quality | Need close collaboration with supplier Higher cost Requires development of use test |
| 5. Lot selection | - | Supply chain disruption and uncertainty Driving inventory cost and complexity |
If you want more insights on resin variability control strategies, download this white paper on understanding and addressing sources of process variability in biopharmaceutical manufacturing.
DOWNLOAD NOWAuthor: Gunnar Malmquist

Dr. Gunnar Malmquist is a Principal Scientist within our BioProcess R&D section and has over four decades of chromatography experience. His current focus is resin design strategies, quality by design, and process analytical technology. Other key areas are biomanufacturing process understanding and creating insights through multivariate data analytics of process and raw material data.
Related literature
- White paper: Quality by design in biotherapeutics purification: understanding and addressing sources of process variability
- Raw material variability: the need for deeper process understanding
- Risk assessment: studying chromatography resin variability
- Infographic: How to ensure process robustness in chromatography