Cytiva further advancing fiber chromatography technology with next release of Fibro technology
- HiScreen Fibro PrismA for mAb purification available for early process development
- Ultrafast purification with HiTrap Fibro PrismA and HiScreen Fibro PrismA enables purification cycle times under 5 minutes compared to hours for resins
- Launch of Fibro technology units for process scale planned during 2021
January 27, 2021
Cytiva is strengthening its new fiber-based protein A platform. HiScreen Fibro PrismA is the newest product available for early mAb purification process development. It complements HiTrap Fibro PrismA, which was launched for research applications in 2020.
Fibro technology enables rapid cycling chromatography to accelerate research and process development, as well as increased productivity in large-scale manufacturing. This creates substantial cost savings and helps bring therapies to market faster. More than 300 academic labs and biomanufacturers have so far selected and successfully used Fibro technology.
Katja Rüger, researcher with Teneobio, says: “Capture purification with Fibro PrismA technology is a lot faster than it is with classic Protein A resin columns, and allows us to speed up our workflow. The product quality is comparable to resin purification and the eluate is more concentrated. This is a great technology that allows rapid protein A purification and saves precious time, while maintaining the product quality we are used to from resin Protein A purification.”
Ryan Davis, senior scientist at Ology Bioservices says: “Fibro PrismA technology could be the largest improvement to mAb platforms over the past decade. Using Ology Bio’s mAb platform, we have gained two orders of magnitude greater productivity using Fibro PrismA than MabSelect PrismA resin.”
The biopharmaceutical market’s shift to developing targeted therapies for smaller patient groups has generated a need to intensify manufacturing processes and make facilities more flexible. Fibro combines the high binding capacity of resins with the high flow rates of membranes in a scalable product that enables hundreds of cycles in a single day for a 20-fold increase in productivity for single-use manufacturing.
Olivier Loeillot, Vice President, Cytiva, says: “We’ve raised the bar with our new Fibro technology. Fibro Prism A represents a step-change in performance that could really accelerate the development and manufacture of monoclonal antibodies. With the introduction of fiber chromatography, our customers can choose between fiber and resin chromatography technologies that offer both high performance and proven reliability.”
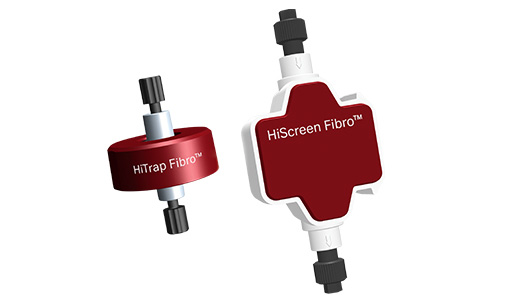
Photo: The first commercially available products are HiTrap™ Fibro PrismA and HiScreen™ Fibro PrismA, compatible with ÄKTA™ chromatography systems.
HiTrap Fibro PrismA and HiScreen Fibro PrismA are compatible with existing ÄKTA chromatography systems and complementary to Cytiva’s resin-based chromatography offering which is widely used in the purification of biopharmaceuticals.
Fibro’s technology features an open porous adsorbent material made of cellulose fibers designed for rapid mass transfer1. Fibro PrismA uses the same chromatography systems, infrastructure, and ligands as resin chromatography, allowing for simple transition into existing biomanufacturing facilities.
Monoclonal antibodies are some of the best-selling biopharmaceuticals with more than 90 mAbs approved by global regulatory agencies as of December 2019 (2). According to GlobalData, the mAbs market is expected to reach a value of $156 billion in 2020, growing at a CAGR of 10% between 2019 and 20252.
For more examples and background, visit Cytiva’s Fibro solutions web page.
1 Cytiva (2019) Overcome chromatography challenges with fiber adsorbents
2 GlobalData (2020) Drugs Database
About Cytiva
Cytiva is a global life sciences leader with more than 8,000 associates across 40 countries dedicated to advancing and accelerating therapeutics. As a trusted partner to customers that range in scale and scope, Cytiva brings speed, efficiency and capacity to research and manufacturing workflows, enabling the development, manufacture and delivery of transformative medicines to patients.
Media Contact:
Colleen Connolly
[email protected]