Size exclusion chromatography (SEC) is the go-to analytical method in the analysis of therapeutic protein drugs. This article describes the factors affecting the reproducibility of columns and how you can minimize column variation.
What factors affect column reproducibility?
Size exclusion chromatography (also known as gel fitration) is a well-established protein analysis method in drug quality control (QC). SEC aggregate analysis is a standard method for analysis of impurities such as mAb aggregates, which negatively affect the quality of protein-based drugs.
Multiple factors affect the performance of your column, the most important being the quality of the chromatography resin and the column packing procedure. However, there are other factors you may need to consider for the reproducible performance of your column.
Column reproducibility is a matter of robust column packing
Development of methods is usually carried out with a limited number of column lots. To feel confident that the results will be reproducible, you need to choose columns with high lot-to-lot consistency. This can be most easily achieved by selecting a good-quality prepacked column.
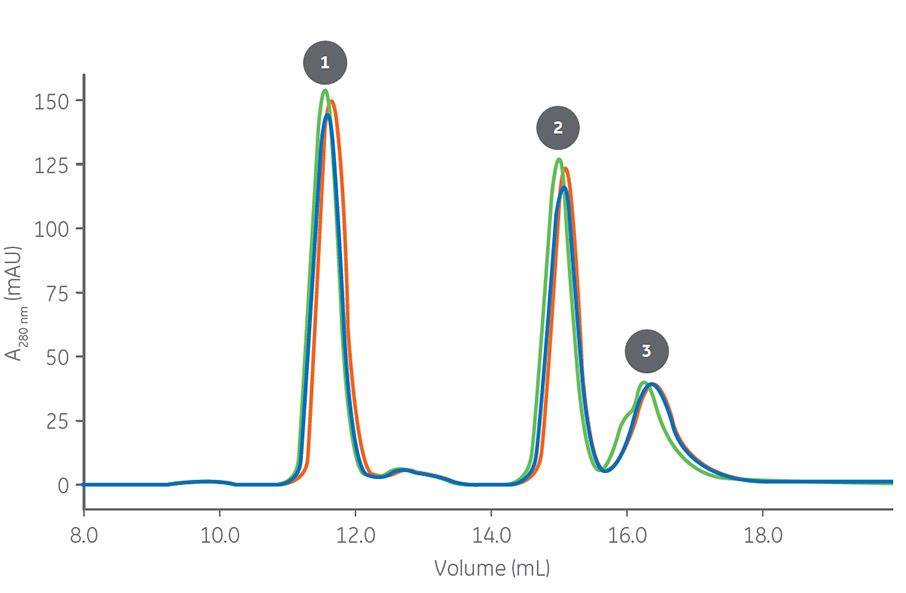
A good example of how excellent column lot-to-lot reproducibility is achieved is shown in Figure 1. Three SEC columns from different lots, which were packed with the same resin, were tested for reproducibility when purifying a mAb, fragment-antigen binding (Fab) region, and single-domain antibody (dAb). The results show very high reproducibility, which is the consequence of high packing robustness.
Columns: Superdex 200 Increase 10/300 GL
Sample: 1. mAb5 (monomer) – Mr 150 000
2. Fab (mAb5) – Mr 50 000
3. dAb (mAb 5) – Mr 13 000
Sample volume: 50 μL (0.2% of column volume [CV])
Buffer: 20 mM NaH2PO4, 300 mM NaCl, pH 7.4
Flow rate: 0.75 mL/min
Fig 1. Three column lots of an SEC column show high lot-to-lot reproducibility of column packing.
Resin lot-to-lot reproducibility is all about narrow specifications and robust production procedures
The narrow manufacturing specification for particle size range and selectivity of SEC resins results in low resin lot-to-lot variation. This gives high consistency and reproducibility, if combined with high-quality packing.
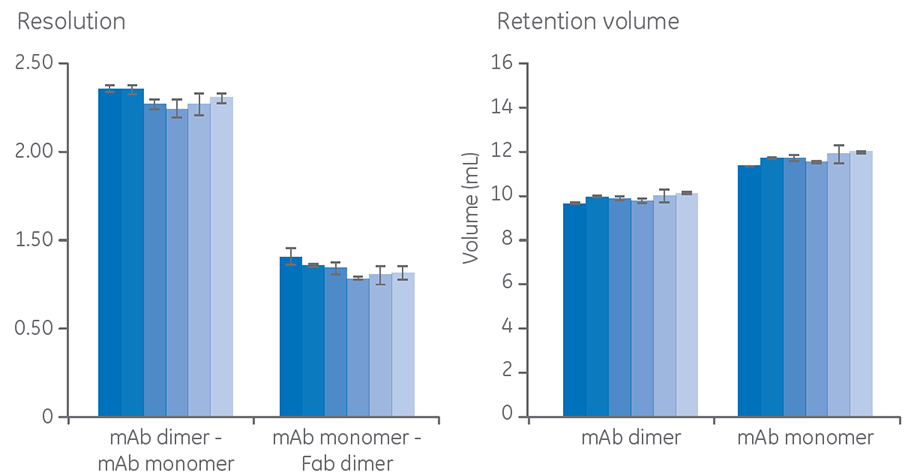
In Figure 2, six resin lots of a SEC resin were compared side by side, and resolution and retention volume were determined for each resin lot. The relative standard deviations (RSD) between the resin lots were < 6% for resolution and < 10% for retention volume, respectively. These results indicate high reproducibility in the manufacturing of the resin as well as in the packing of the columns.
Columns: Superdex 200 Increase 10/300 GL
Sample: mAb5 containing monomer, dimer, and Fab fragments
Sample volume: 50 µL (0.2% CV)
Buffer: 20 mM NaH2 PO4, 300 mM NaCl, pH 7.4
Flow rate: 0.75 mL/min
Fig 2. Comparison of six different batches of resin on resolution and retention volume in the purification of mAb, mAb aggregates, and fragments.
Choose columns that are robust over time
Besides lot-to-lot consistency, your column performance needs to be consistent with reuse over time. Use regular cleaning with NaOH to restore the column performance and prolong its lifetime.
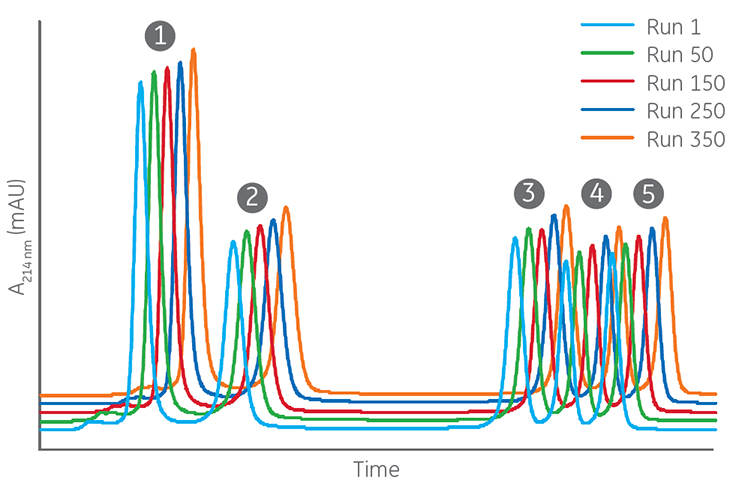
In Figure 3, a total of 350 injections of a sample mix consisting of low concentrations of proteins and peptides was performed on a high-resolution column. Peak areas and resolution were essentially unchanged during the study, which shows that the column is robust and several runs can be performed with consistent performance. By adding a NaOH cleaning step after 10 to 20 runs (depending on the origin of the sample), the lifetime of the column can be prolonged even further.
Columns: Superdex 30 Increase 10/300 GL
Sample: 1. Cytochrome C (Mr 12 400), 0.16 mg/mL
2. Aprotinin (Mr 6500), 0.16 mg/mL
3. [Ile7]-Angiotensin III (Mr 897), 0.08 mg/mL
4. Triglycine (Mr 189), 0.16 mg/mL
5. Glycine (Mr 75), 5.6 mg/mL
Sample volume: 50 µL
Buffer: 20 mM phosphate buffer, 280 mM NaCl, pH 7.4
Flow rate: 0.8 mL/min
System: HPLC
Fig 3. Repeated injections of a sample mix consisting of proteins and peptides on a high-resolution SEC column. Results from run 1, 50, 150, 250, and 350 are shown. Peaks of the proteins and peptides are labeled 1 to 5.
Summary
Bear in mind that the lot-to-lot consistency of a SEC resin impacts on the analysis results over time. Pick a resin and prepacked column that have a high lot-to-lot reproducibility. Prepacked columns packed in a controlled environment minimize the variation between lots.
In QC and in research, the same analysis method is usually used during a long period of time using the same products. Therefore, several column lots may be used over time, which requires high lot-to-lot reproducibility.