Biomanufacturing requires large volumes of buffers, and preparation of these liquids can quickly become a bottleneck. Here, two options that can help intensify your large-scale buffer management are presented.
Outsource buffer production
Expanding buffer capacity often requires major capital investments. Letting an expert supplier deal with buffer preparation can be a cost-efficient alternative. Outsourcing will save you time and cost for raw material validation and characterization, while giving you access to the supplier’s manufacturing capabilities, supply chain, logistics expertise, and QA/QC teams. The initial vendor validation work can be intense, but once this has been done, you can benefit from a robust supply chain, reduced buffer preparation time, reduced quality control testing time, and reduced quality assurance documentation.
Outsourcing can also be a way to add extra resources to your internal buffer capacity. Whereas some biomanufacturers prefer to outsource complex buffers, possibly with hazardous components, and prepare simple buffers internally, others may wish to be in control of the complex buffers and instead purchase large volumes of more simple fluids such as NaCl, NaOH, or ethanol. Yet other manufacturers prefer to keep the main buffer preparation in house, while validating a vendor as a second supplier to reduce risk and gain extra capacity at times of constraints in buffer preparation.
Intensify processes with in-line buffer preparation
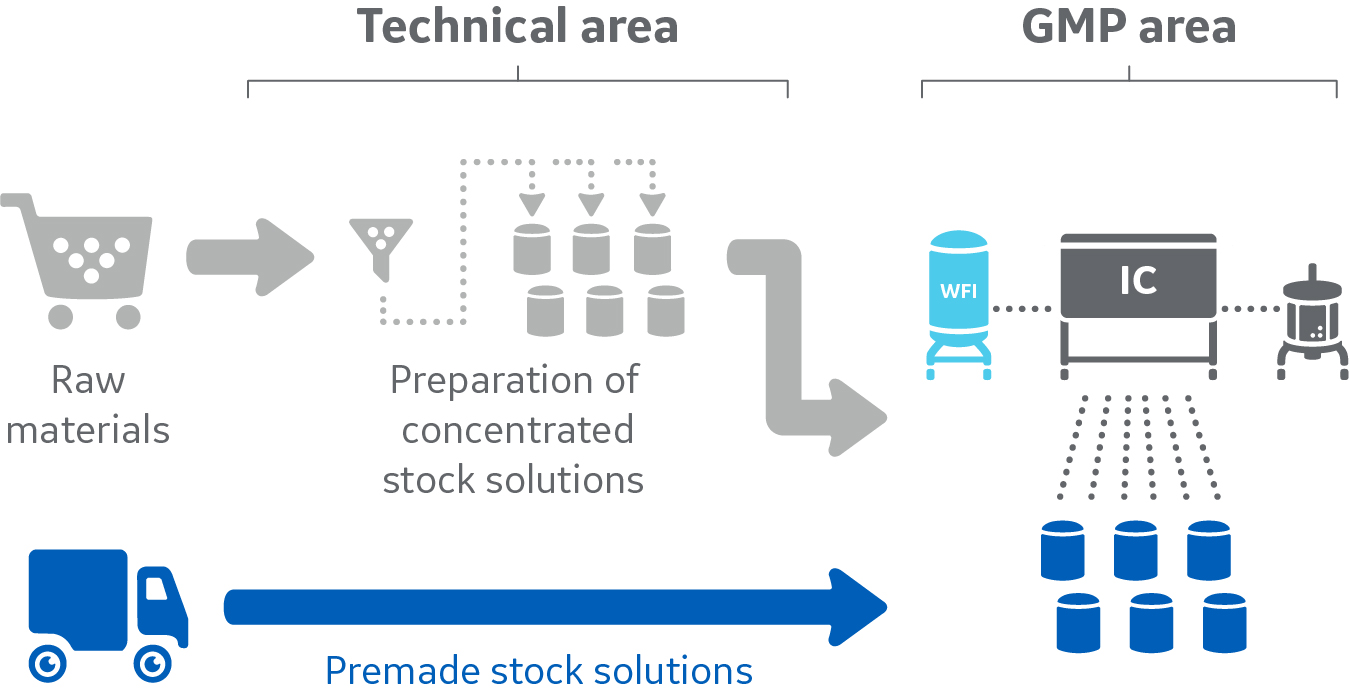
Implementing techniques such as in-line dilution (ILD) and in-line conditioning (IC) is another way of meeting buffer challenges. In this case, buffer production remains an in-house activity, but labor and space required for preparing and handling the buffers are greatly reduced. Depending on the chosen solution, the process can be further optimized by integrating the buffer preparation process with the chromatography unit operation, eliminating the need for intermediate storage in buffer bags or hold tanks. In addition to the reduced floor space requirement, automating the preparation process will also reduce the need for manual handling, freeing up human resources and decreasing the risk of human error.
As buffers are used to maintain purification conditions as well as stabilize the bioproduct and preserve its functional characteristics, correct buffer formulation is crucial for success in bioproduction. Using techniques such as IC will not only help to automate the process, but also ensure accurate formulations through in-line feedback regulations of the final buffer.
Take it one step further: combine outsourcing with in-line buffer preparation
Both outsourcing of buffer preparation and implementation of technologies such as ILD and IC help release resources to other activities that add more value in biomanufacturing, and both these solutions are described in more detail in the whitepaper Buffer management solutions for large-scale biomanufacturing applications. To further intensify the buffer preparation process, you can combine outsourcing with in-line buffer preparation. By outsourcing preparation of the input concentrates, time is not only saved in manual preparation of these concentrates, but also in the reduced logistics and raw material handling.
Combining outsourcing with in-line buffer preparation enables great time-savings and enhances resource utilization.
Sign up for future post that will discuss the following topics:
- Batch versus continuous bioprocessing.
- Single-use versus stainless steel equipment in downstream bioprocessing.