Challenges in the development of safe biotherapeutics and how to overcome them with a robust analytical strategy
In recent years, the global biopharma industry has seen a rapidly advancing trend toward biotherapeutics or “biologics drugs”—drugs that are produced from living organisms or contain components of living organisms.(1) In 2018 alone, the U.S. FDA approved 17 biologics out of a total of 59 drugs – the highest number to date.(2)
Due to their ability to effectively treat complex health problems like cancer, infectious diseases, and autoimmune diseases, biologics have become a major focus for most pharmaceutical companies.(3) Additionally, the Food and Drug Administration (FDA) facilitates approval of many biologics through expedited programs, making the ventures even more lucrative to Big Pharma. Last year, six of the 15 best-selling drugs were biologics.(4)
This article will delve into why the pharma industry is heading toward a biologics revolution, what challenges exist when developing these drugs to be efficacious but safe, and how novel analytical techniques can help circumvent these issues and optimize development.
Why the transition to biologics like bispecific antibodies, antibody-drug conjugates, and other immunoconjugates?
The emerging new generations of biologically engineered antibody drugs are driving a strong shift from discovery and production of standard monoclonal antibodies (mAbs) to multispecific formats, such as bispecific and trispecific antibodies, antibody fragments, antibody-drug conjugates (ADCs), and other immunoconjugates.
According to Fredrik Sundberg, Ph.D., global director of strategic customer relations at Cytiva, Uppsala, Sweden, the main reason for this is that “they offer higher potency at smaller dosages, which translates to smaller production volumes.”
Essentially, developers get a bigger bang for their buck, so to speak, with complex, next-gen biotherapeutics. “Blocking two target antigens with a single drug molecule instead of two can increase efficiency and even the dosing requirements,” Sundberg remarks.
“Bispecific antibodies (BsAbs) are used in cancer immunotherapy because binding multiple epitopes with a single antibody offers several benefits,” says Sundberg. “For example, one specificity can directly bring immune cells closer to target cancer cells, or deliver a drug to the target, while another could target individual molecules.”
BsAbs are designed to target and interact with two different surface antigens simultaneously. Thus, they provide higher binding specificity relative to monospecific Abs and can also deliver higher cytotoxicity by directing specific effectors of the immune system to tumor cells.(5)
In the case of ADCs, antibodies are bound to cytotoxic drugs and are used as carriers to transport these toxic payloads directly to target tumor cells. “They bind like missiles, targeting and releasing the toxic payload and killing cancer cells,” explains Sundberg.
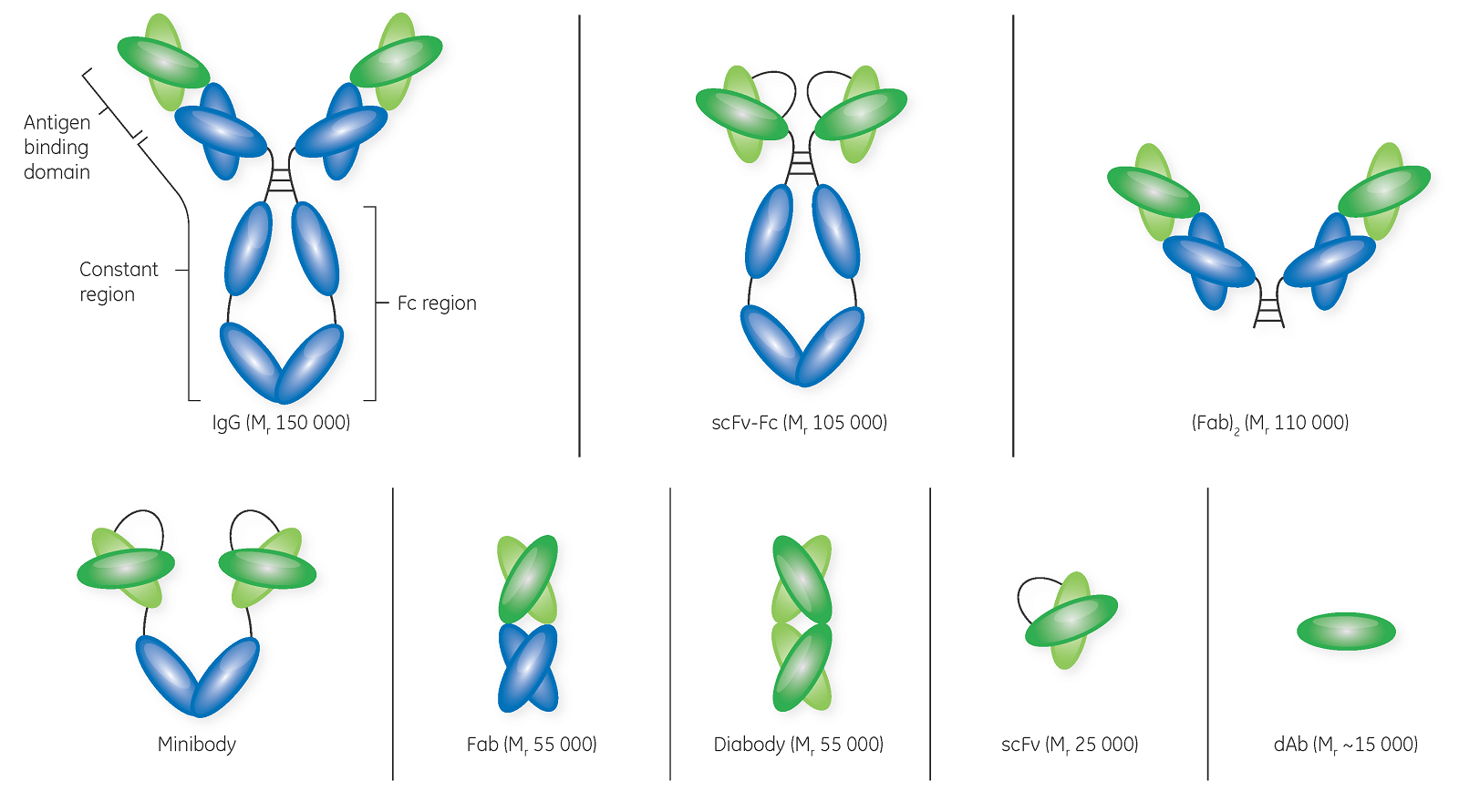
Researchers are now combining formats like BsAbs and ADCs to create more innovative and complex structures with vastly improved specificity and potency in the treatment of various cancers.(6) (Fig 1) But as complexity increases, so do the challenges in development and concerns regarding patient safety.
Fig 1. Protein engineering technologies are producing increasingly diverse therapeutic proteins—whole antibodies, antibody fragments, and fusion proteins (e.g., bispecific antibodies).
What are the challenges associated with developing safe next-gen biologics drugs?
Next-gen biotherapeutics hold great promise as effective drugs, but they also come with a fair share of challenges during engineering, development, analytical characterization, and manufacture. “The differences of complex biologics can affect everything from how difficult the molecules are to manufacture and their stability in that process, to how well patients will eventually tolerate these medicines,” says Sundberg.
Immunogenicity:
Immunogenicity is defined as the ability, or the degree, to which a particular substance may provoke an immune response and production of antibodies.(7) Commercial consequences of immunogenicity for pharmaceutical and biotech companies could range from negligible to severe, depending on the nature of the effect, the type of product, and the size of the company. There are also wide-ranging implications for patients, physicians, and payers.
“As you add more complexity to the biologic in terms of structure and function, they change from a ‘standard natural antibody’ produced by the body’s defense to an engineered one which will be a completely foreign entity to the body and immune system,” explains Sundberg. “This leads to immunogenicity and concerns in patient safety—one of the biggest challenges today for drug developers.”
For example, single-target antibodies are close to naturally existing molecules, so the body often accepts them with few side effects. But the more complexity you add to the molecules (e.g., creating bi-/multispecific antibodies and conjugating toxic entities), the more likely it is that the body will reject them, leading to unwanted side effects and immune responses that are hard to predict and test.
Furthermore, developers need to pay extra care to omit unwanted side products during purification of these complex biologics. But some byproducts that may cause toxicity are hard to separate, making large-scale production difficult if one were to use a standard mAb platform approach. Even though manufacturers have found ways around this now, monitoring for immunogenicity in patients remains a regulatory requirement.
Stability issues:
Biological activity of antibody-based therapeutic molecules is closely related to their chemical and structural stability. But these complex biomolecules are not very stable and can easily degrade. As such, maintaining stability of complex antibodies is another major issue for developers.
Physical and chemical degradation may occur at various stages of the antibody life cycle, from production/purification through formulation, storage, and delivery. Antibody aggregation of monomeric Ab units to dimers, trimers, and larger aggregates is the most common type of physical degradation induced by various stress factors like temperature changes, freezing/thawing, mechanical stress (agitation, pumping, filtration), pH/conductivity change, etc. Chemical degradation occurs via oxidation, deamidation, isomerization, cross-linking, clipping, and fragmentation.(8)
“BsAbs and ADCs can be especially unstable, diverse in their makeup and can have aggregation tendencies and varying impurity profiles,” Sundberg notes. “So, these kinds of molecules are difficult to control chemically and, therefore, produce at large scale.”
Other difficulties in manufacturing and purification of next-generation biologics:
Particularly for ADCs, process conditions can be much more arduous than for “naked” antibodies, as you have to consider the antibody–drug conjugation reaction, in addition to purification challenges. Once the antibody is conjugated to the cytotoxin, it requires further purification (either through chromatography or ultrafiltration/diafiltration). Also, the process needs to be closed and regulated due to the high-level of toxicity that’s not observed with other protein conjugates.
“At this stage in the process, the batch sizes are small, so some manufacturing can be done in a biosafety cabinet, but this can be cumbersome,” says Sundberg. “Here, the use of disposable technology and closed purification systems is of great benefit. Also, the chemical compatibility and leachables/extractables profile of the disposable technologies, particularly the films, must be assessed.”
Another critical quality attribute of ADCs – that essentially defines their potency – is the drug antibody ratio (DAR).(9) This ratio must to be monitored at the beginning, middle, and end of every step in the process to ensure the efficacy of the ADC.
How can developers tackle these issues and perform an optimal characterization?
Christina Burtsoff-Asp, global product marketing manager at Cytiva, believes that the key is “being able to understand whether you’re making changes that deliver the right properties, such as high affinity, safety, robustness, developability.” She notes that, in this sense, understanding the mechanism of action and biological relevance of the therapeutic plays a vital role. “For this, high-quality analytics and data are critical.”
According to Burtsoff-Asp, surface plasmon resonance (SPR)-based systems can provide high-quality molecular interaction data to guide any biotherapeutic development program—from target selection to the final quality control assessments. “But the best approach a developer can take, is to use a combination of techniques—in essence, a ‘toolbox’ of complementary, orthogonal tools that will deliver a comprehensive analysis,” she adds.
Classical chemical analysis techniques, such as mass spectrometry (MS), reversed-phase high-performance liquid chromatography (RP-HPLC), hydrophobic interaction chromatography (HIC), analytical ion exchange chromatography (IEX), and size exclusion chromatography (SEC), have been used for decades and work well for measures like determining DAR in ADCs and evaluating aggregate formation.(9)
Every change on a molecular backbone might impact the ability to interact with the target molecule. This is where label-free optical techniques (e.g., SPR biosensors) can provide many benefits, including unique insight into binding events.(10)
“With Biacore™ SPR systems, you can monitor critical binding kinetics and conduct epitope binning to characterize antibody structure and function throughout the entire workflow,” Sundberg says. “You can check each modification made to an ADC(11) and assess how it affects binding.”
SPR-based assays also allow researchers to assess the binding activity of bsAbs in a single setup, with either a “bridging assay” or “dual-binding assay” formats.(12) These innovative methodologies simplify analysis and are useful for avoiding several combinations of classical immunoassays (e.g., enzyme linked immunosorbent assay, ELISA) to assess binding. They can indicate function loss in a parallel analysis of two interactions at the same time, rather than with two different ELISAs.(13)
“Biacore SPR systems can be used as an end-to-end solution or platform technology, from early drug discovery through to quality control, even for more complex biologics,” Sundberg emphasizes. “In other words, this makes assay validation and transfer easier and accelerates the up/downstream processes, as well as overall time to market.”
Addressing immunogenicity and patient safety issues
Continuous assessment of immunogenicity in patients is a regulatory requirement with novel, complex biologics, because it can impact the safety profile of a drug and its efficacy. Concerns about immunogenicity are serious issues; they may cause a physician to switch or terminate treatment with consequential impact on the drug’s commercial potential. As such, developers must have the right technology to screen patients from Day 1 of the treatment.
For this, traditional techniques like ELISA immunoassays, chemiluminescence assays, and cell-based assays can be used. But they may not be effective in delivering results quickly (i.e., incubation and wash steps can take time and induce complications) or with better insight into molecular interactions.
According to Sundberg, SPR can be used as a great complementary, orthogonal analytical method, providing high-quality, content-rich data in real time. The SPR-based systems, such as Biacore 8K series and Biacore T200, allow researchers to conduct a comprehensive assessment of serum antibodies, including:
- assessing unwanted immunogenicity, such as detecting early low-affinity immune responses, characterizing antibody isotypes, and monitoring class switches
- performing comparative immunogenicity assessment of biosimilars (which enables accurate measurement of similarity)
Addressing efficacy and stability of biologics during development
Efficacy:
Acquiring reliable kinetic parameters to characterize protein-protein interactions is essential for ensuring high efficacy of a drug. However, it’s easier said than done – assessing binding affinity and kinetics of high-affinity antibodies can be a strenuous task.
SPR is the current "gold standard" for real-time detection of antibody-antigen binding and kinetics; it has been used for about three decades to enable early selection of criteria-meeting therapeutic antibody candidates.(14,15)
Sundberg explains that sensitive SPR-based instruments with continuous flow fluidics are conducive for optimizing kinetic studies, “as this type of setup is better at resolving high-affinity interactions with slow dissociation rates.”
For example, Biacore 8K series and Biacore T200 enable:
- rapid screening and characterization of high-affinity binders
- binding studies of immobilized Fc receptors such as Fc-gamma receptors, neonatal Fc-receptors, or complement binding
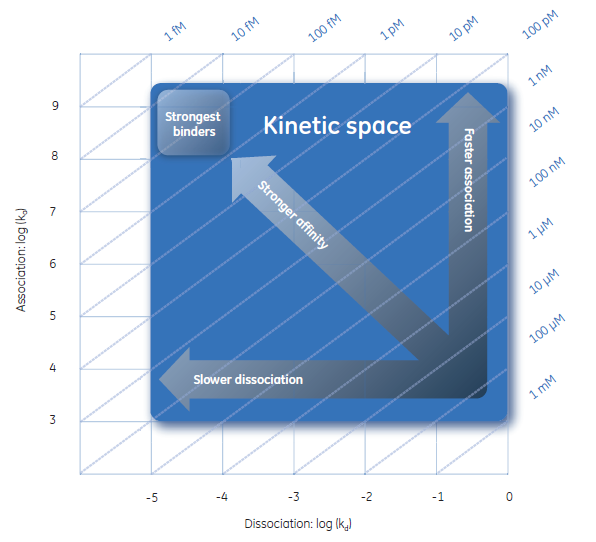
The high sensitivity of SPR technology makes it possible to obtain a confident kinetic analysis over a wide dynamic range, from very fast on-rates to very slow off-rates. (Fig 2) “You can clearly differentiate between stable binders and even determine very slow off-rates reasonably well,” adds Sundberg. “High sensitivity also allows one to analyze low abundance molecules or sensitive, complex targets.”
Fig 2. With the expanded kinetic space of the Biacore systems, high-quality information on the most relevant biomolecular interactions, even at the extremes of kinetic behavior, can be confidently measured. It is possible to precisely determine the true dissociation rates of strongly binding antibodies, when refining drug efficacy. It is also possible to differentiate between the fastest binders, which can help select better candidates for biological processes limited by bioavailability.
SPR biosensors can also provide an affinity ranking of antibody candidates by rapidly screening the binding of crude supernatants and determining binding constants of purified preparations. SPR biosensors can further differentiate the functional activity of lead candidates via epitope binding studies.
Antibodies from multiple epitope bins may imply different mechanisms of action. This can be particularly advantageous when pursuing oligoclonal therapies for cancers or infectious diseases that target more than one biological pathway simultaneously.
“The identification of unique and diverse epitopes is not only of importance for discovering antibody binding pairs (e.g., immunoassays), but may also broaden the potential for intellectual property protection,” notes Sundberg.
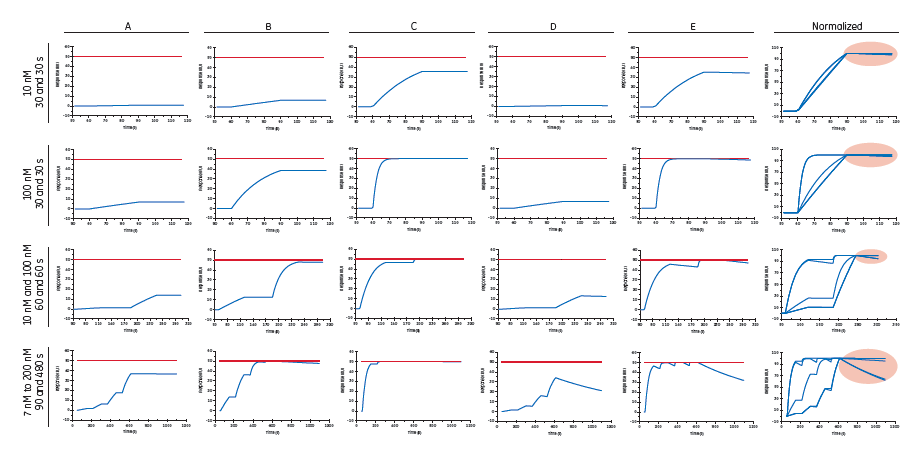
Fig 3. Different antibody screening and characterization approaches: antigen is injected at various concentrations and for varying time as indicated to the left. Conditions A to E correspond to different rate constants (numbers not disclosed). The red horizontal line in A to E plots indicates the saturation response. The normalized plots to the far right were obtained by rescaling sensorgrams on a percentage scale and are intended for comparison of off-rates as indicated by the red ovals.
Stability:
Successful production of antibody-based therapeutics also requires careful assessment of the various degradation pathways possible for a molecule (both physical and chemical) and implementing control over those pathways. Each of the steps in recovery and purification must be optimized, based on the process requirements and the molecule characteristics, to ensure a robust, stable, and scalable production.
Amino acid modifications and changes in glycosylation can be identified by a combination of analytical techniques such as mass spectrometry (MS), electrophoresis, and chromatography, in which product variants can be isolated and characterized.
Changes in target binding and neonatal Fc receptor (FcRn) binding for stressed samples indicate that interaction analysis may be used to detect small changes in antibody reactivity. Target and FcR interactions can thus play an important role in stability and forced degradation studies.“There are examples where SPR-based assays have been used for the rapid analysis of changes in binding reactivity related to target-, FcR-, and IgG domain-specific functionalities,” notes Sundberg. “These cases strengthen the use of Biacore analysis for screening of changes in reactivity enforced degradation studies and in regular stability testing.”
The on-demand webinar “Principles of immunogenicity assessment using Biacore T200 SPR system” provides more detailed information on how SPR can be applied for immunogenicity testing.
Conclusion
For an optimal approach to characterization and immunogenicity assessment, developers should use an orthogonal analytical strategy combining conventional technologies with newer, label-free optical methods.
Innovations in manufacturing and analytical technologies are imperative, not only to develop novel biotherapeutics that are safe and efficacious drugs, but also to establish predictable, reproducible, and economical production bioprocesses.
View Principles of immunogenicity assessment using Biacore T200 SPR system webinar.
References:
- Whatare “biologics” Questions and Answers, U.S. Food and Drug Administration.
- Mullard,A. 2018 FDA Drug Approvals, Nat. Rev. Drug. Discov., (2019).
- GlobalBiologics Market: High Profitability and Increased Profit Margins through Premium Prices Encourage Growth. Transparency Market Research (2018).
- Philippiddis,A. Top 15 Best-Selling Drugs of 2018. Genetic Engineering & biotechnology News, (2019).
- Sedykh,S. E. et al. Bispecific antibodies: design, therapy, perspectives. Drug Des. Devel. Ther., 12, 195–208, (2018).
- de Goeij,B. E. et al. Efficient Payload Delivery by a Bispecific Antibody–Drug Conjugate Targeting HER2 and CD63. Mol. Cancer Ther., 15, 2688–2697 (2016).
- Lagassé,H.A.D. et al. Recent advances in (therapeutic protein) drug development [version 1; peer review: 2 approved]. F1000Research (2017).
- Ross,P. L. et al. Physical and Chemical Stability of Antibody Drug Conjugates: Current Status. J. Pharm. Sci., 105, 391–397 (2016).
- Tang,Y. et al. Real-Time Analysis on Drug-Antibody Ratio of Antibody-Drug Conjugates for Synthesis, Process Optimization, and Quality Control. Sci. Rep., 7, 7763 (2017).
- Karlsson,R. et al. Comparison of surface plasmon resonance binding curves for characterization of protein interactions and analysis of screening data. Anal. Biochem., 502, 53–63 (2016).
- Healey,G., D. et al. A RAGE-Targeted Antibody-Drug Conjugate: Surface Plasmon Resonance as a Platform for Accelerating Effective ADC Design and Development. Antibodies, 8 (1), 7 (2019).
- Meschendoerfer,W., et al. SPR-based assays enable the full functional analysis of bispecific molecules. J Pharm. Biomed. Anal., 132, 141–147 (2017).
- Gassner,C. Development and validation of a novel SPR-based assay principle for bispecific molecules. J Pharm. Biomed. Anal., 102, 144–149 (2014).
- Yang,D., et al. Comparison of biosensor platforms in the evaluation of high affinity antibody-antigen binding kinetics. Anal. Biochem., 508, 78–96 (2016).
- Cooper,nbsp;M. A., .Optical biosensors in drug discovery. Nat. Rev. Drug Discov., 1, 515–528 (2002).