Which parameters really matter for cell therapy process development? G. John Morris discusses the evidence in this blog post.
Need for science-based cryopreservation and cell thawing
It is an invigorating time to be involved in cell therapy development. People do fantastic science on the cell biology, fine-tuning the CAR Ts and TCRs, growing the cells, all of those parameters. But when it comes to freezing and thawing T cells, there is not much real science. We are trying to simplify freezing and thawing protocols, basing them on biology and physics. It is important to define these final steps so as not to waste the time, effort, and money invested to that point. Here we delve into the science of cell thawing.
Thawing T cells after cryopreservation
How critical is quick thawing when working with mammalian cells, such as T cells used for cellular therapies? There is a general dogma in the cell therapy industry that cells must be thawed very rapidly, otherwise they will be damaged. This belief imposes limits on process development for clinical samples. We committed to understanding whether quick cell thawing was indeed a requirement driven by science.
The existing literature, which is quite outdated, was a bit contradictory and confusing. In collaboration with the Cell and Gene Therapy Catapult in London, we decided to do an experimental program, just to critically define what is important. We looked at cooling and warming T cells at different rates, and we looked at the viability.
Rapid thawing myth debunked
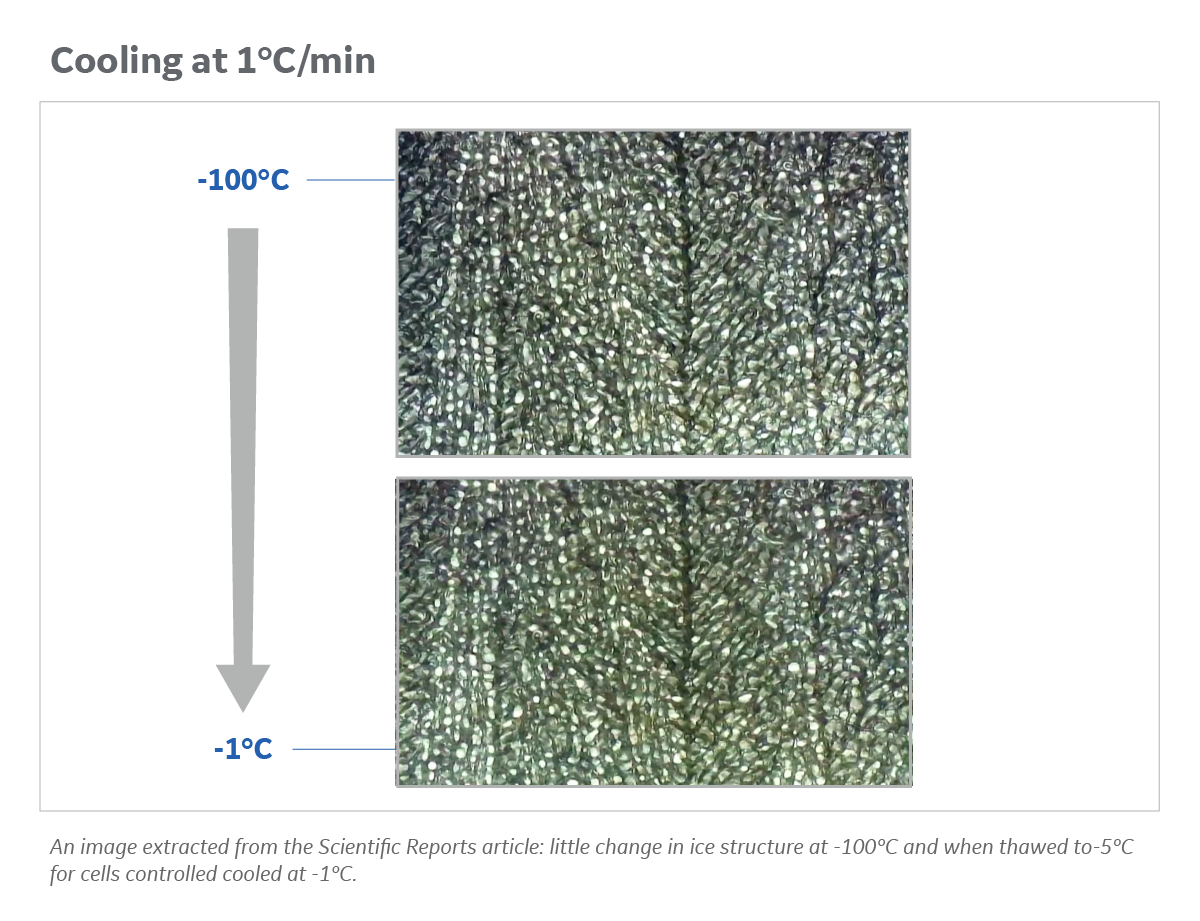
We got some interesting results, published in Nature journal Scientific Reports, using both water baths and dry thawing methods. If you cool relatively slowly, which most people would do in this industry (1 degree/minute or even less), the rate of cell thawing is not critical. It is only when you cool rapidly that you have to start warming rapidly. We then started to investigate some of the physical reasons that was important. We looked at the physics of the ice crystallization process using specialized microscopy. In fact, that showed if you cool slowly there is no effect on the ice crystals when you warm up – they do not recrystallize. But when you cool rapidly they do recrystallize, and that is probably one of the things that leads to damage. Next, we looked at the whole freezing process using a completely different analytical technique where we did differential scanning calorimetry (DSC) with some collaborators at INRA in Paris. The DSC results validated the pictures we took and the viability data. Figure 1 shows an example of ice structure before and after thawing.
Fig 1. Ice structure and changes observed during warming cycle at 37°C. For cells control-cooled at 1°C/min, there is little change in ice structure at -100°C and when thawed to -5°C.
Why it matters for cell therapy process development
In a laboratory you would probably put something into a warm water bath, and it would thaw pretty rapidly. However, in clinical applications or if you are in a clean room, you cannot use a water bath, which is a potential source of contamination. So you have to look at other ways of thawing. There is nothing as efficient as putting things in warm water. Water shapes the vessel and is a very good heat transfer material. Everything you do in process development where you do not go into a water bath is going to be slower, and people are going to get concerned that is going to give them problems.
Our work provides the evidence that although dry thawing is a little bit slower than a water bath, that is absolutely fine. The other thing it tells you is if you really want to go to larger volumes or different format containers, the rate of thawing is not going to limit the use of those containers. Again, there is lots of thinking in the industry that if you go to a larger bag or a larger volume, then you are going to run into problems because thawing is slower. In fact, you can go to larger volumes without having concerns that you will not be able to thaw rapidly enough.
From a process development point of view the take-home message is that the rate of thawing is not critical. What is critical is that when you thaw out you should not allow the sample to become warm. With a water bath there is a danger that if you leave samples in there too long they will warm up, and then you get damage.
Read more tips on cryopreservation and cell thawing.