You asked us about analytical size exclusion chromatography (SEC) quality certificates, compatibility with HPLC systems, considerations for column selection, and more. Here are the answers.
A: Yes, GE’s Superdex Increase and Superose Increase columns are used on traditional HPLC systems for analytical purposes, for example, quality control. Standard 1/16″ (fingertight) connectors can be used.
Check this list of recent publications where GE’s Superdex Increase and Superose Increase columns have been used for analytical SEC.
Q: Do I need extra connectors to attach the Superdex Increase or Superose Increase columns to HPLC systems?
A: No, standard 1/16″ (fingertight) connectors are used on Superdex Increase and Superose Increase columns and they are compatible with traditional HPLC systems.
Check this list of recent publications where GE’s Superdex Increase and Superose Increase columns have been used for analytical SEC.
A: To find the CoA for your column, enter the column lot number in the search field. The certificate includes information about the resin lot packed into the column. It also shows that the individual column meets our specifications for efficiency and asymmetry.
A: The difference in HETP (efficiency value) is most likely dependent on differences in your system setup compared to the test system at GE. The efficiency of our columns is tested in an optimized chromatography system with minimal internal system volumes. The same efficiency may not be possible with other systems.
The recommended procedure is to set a reference value by testing the efficiency on the new column in a specific system. Then you can check the quality of the column performance over time based on that number.
A: When choosing SEC columns for protein analysis many parameters need to be considered. Resolution is one of the most important and it is influenced by several factors including:
- Resin properties, like resin chemical composition, particle (bead) size and size distribution, pore size and selectivity, and fractionation range
- Column-related factors, like column length and column packing efficiency
- Running conditions, like sample volume and injection technique, buffer composition, and flow rate
- Chromatography system configuration, like tubing diameter and flow path volume
After the selection of SEC resin, column dimension and sample volume are the two factors that affect the resolution of the separation the most.Download a white paper on protein analysis with SEC.
A: Different resins have different fractionation ranges and are thus able to separate molecules in different size ranges. Figure 1 shows the fractionation ranges and some typical application areas for the four Superdex Increase and Superose Increase resins. These resins are available in three different column sizes to fit different purposes.
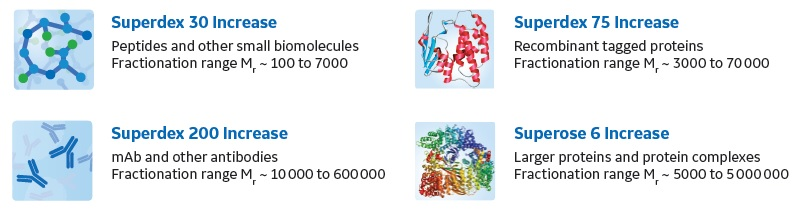

Fig 1. Superdex Increase and Superose Increase resins offer high-resolution SEC separations in prepacked columns. Note that Superdex 30 Increase is not available packed in 5/150 column as a standard product.
For quality control (QC) purposes, we recommend using the 10/300 GL column format.
Learn more about Superdex 30 Increase
Learn more about Superdex 75 Increase
Learn more about Superdex 200 Increase
Learn more about Superose 6 Increase
Q: Are Superdex Increase and Superose Increase columns well-suited for separation of large, sensitive biomolecules?
A: Superose 6 Increase has a fractionation range of 5000 to 5 × 106 (~ 3 to 25 nm) and can be used for molecules in this size range. The molecular weight range is based on globular proteins. The separation range for other biomolecules with other structures might be different.
The fractionation range for Superose 12 is covered by four resins: Superose 6 Increase, Superdex 200 Increase, Superdex 75 Increase, or Superdex 30 Increase (Fig 2). We recommend that you choose the one that best fits the size of your target molecule.
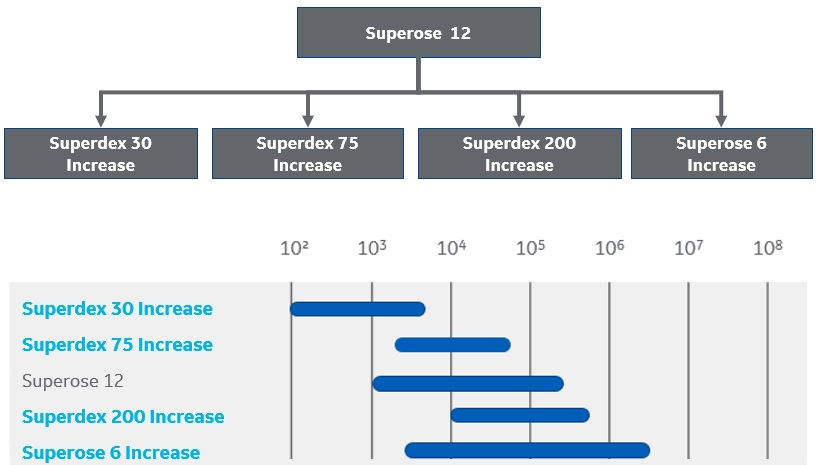
Fig 2. Resins that can be used instead of Superose 12.
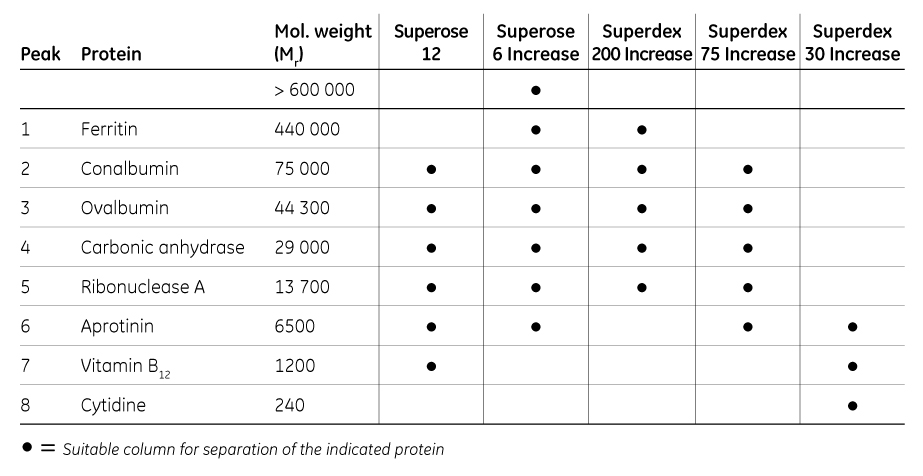
As a guide to where to start, we have compared a run with the same eight molecules on Superose 12 and the four Superose Increase and Superdex Increase resins. The peak numbers shown in the chromatogram (Fig 3) correspond to the proteins shown in Table 1.
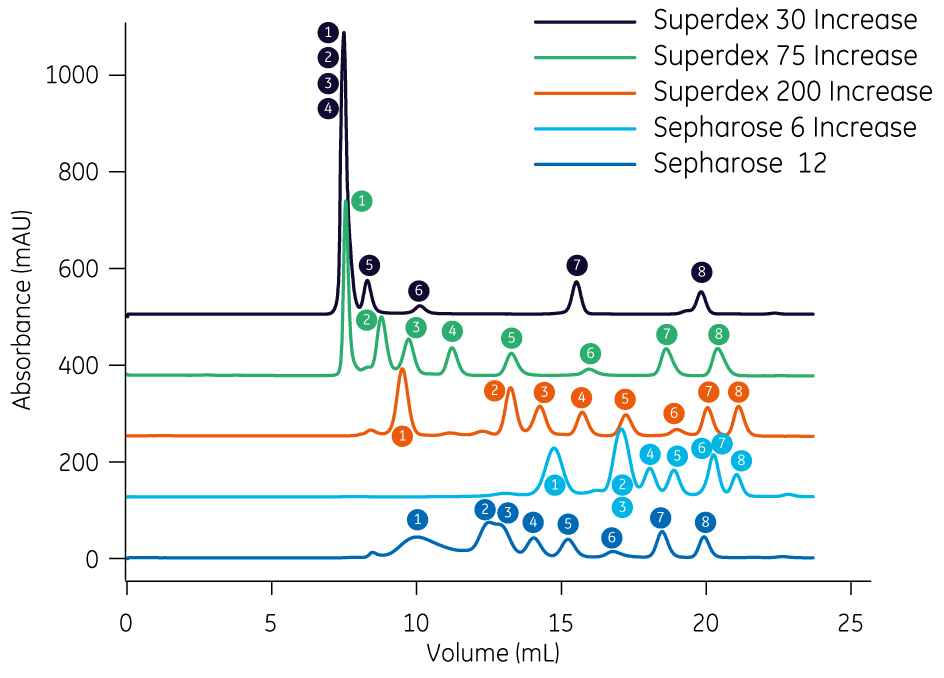
Fig 3. Guide to selecting a replacement resin for Superose 12. Chromatograms showing separation of eight molecules on Superdex 30 Increase, Superdex 75 Increase, Superdex 200 Increase, Superose 6 Increase and Superose 12. Peak numbers correspond to peaks listed in Table 1.
Table 1. Guide to selecting a replacement resin for Superose 12
A: Pore size is a complex function of the pore diameter, the pore shape, the number of pores, and pore connectivity. All pores are not equal even within a single chromatography particle. It is therefore an oversimplification to describe a chromatography resin’s pore size with a single number such as average pore size diameter. GE does not measure pore size for chromatography resins.
The relationship between resolution and selectivity for a SEC resin is described in the selectivity curve. The steeper the selectivity curve, the higher the resolution that can be achieved. Resolution is also affected by band-broadening, which is dependent on the bead size of the SEC resin.
Download the white paper Protein analysis with size exclusion chromatography to learn more.
The most important parameter to consider for quality control, after checking that high enough resolution can be achieved, is the lot-to-lot consistency both in terms of column to column and resin batch-to-batch.
Column-to-column reproducibility
Results from tests of column packing robustness are shown on Superdex 200 Increase 10/300 GL columns in Figure 4. Three columns from different lots, packed with the same resin lot, were tested and the results show very high reproducibility.
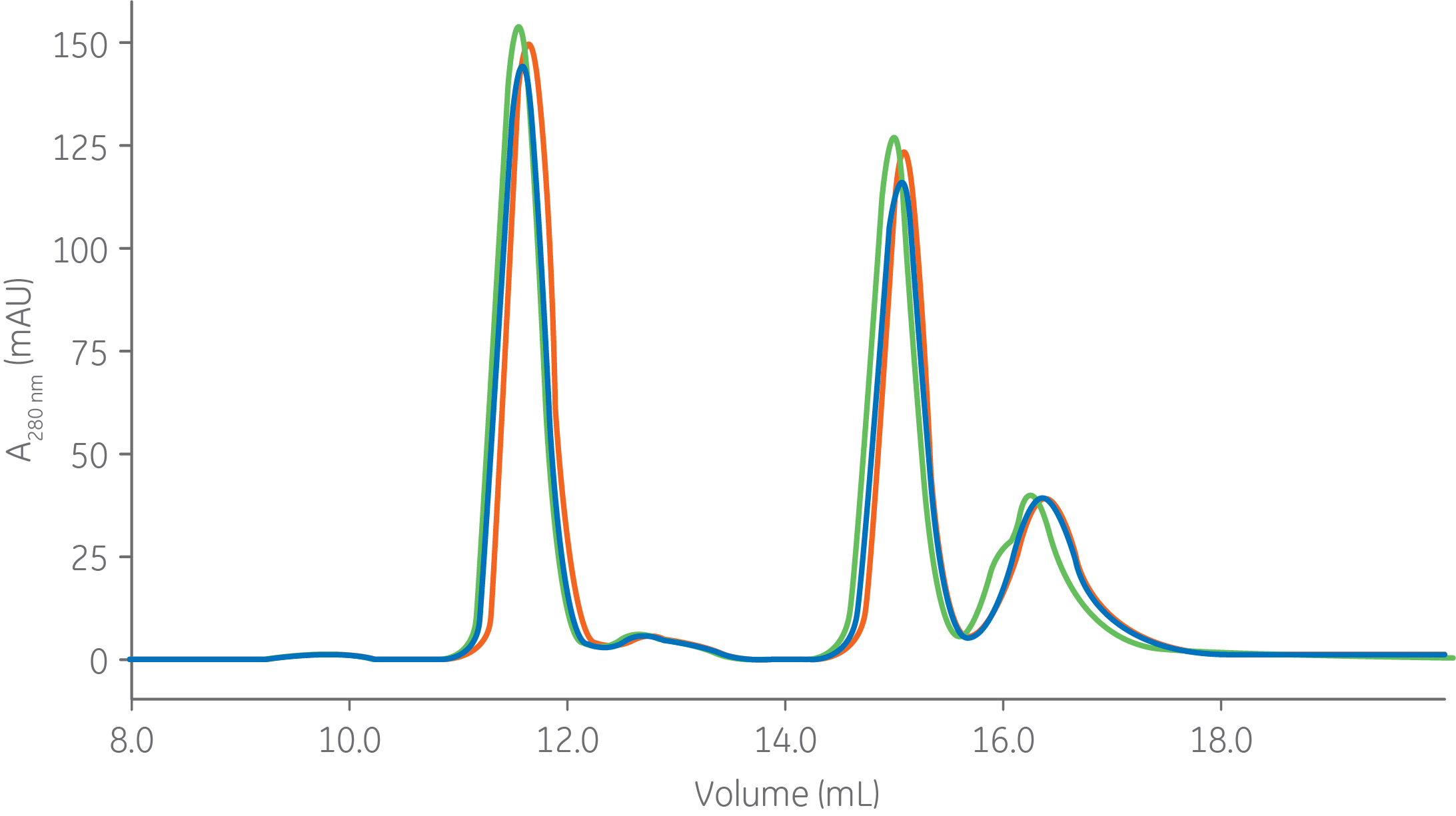
Fig 4. Three column lots of Superdex 200 Increase 10/300 GL show high lot-to-lot reproducibility of column packing. Column: Superdex 200 increase 10/300 GL, Sample: 1. mAb5 (monomer) Mr 150 000, 2. Fab (mAb5) Mr 50 000, 3. dAb (mAb 5) Mr 13 000, Sample volume: 50 µl (0.2% CV), Buffer: 20 mM NaH2PO4, 300 mM NaCl, pH 7.4, Flow rate: 0.75 mL/min
Batch-to-batch reproducibility
Results from batch-to-batch reproducibility of different resin lots of Superdex 200 Increase are shown in Figure 5. Six different resin lots were compared for resolution and retention volume and show minor differences in relative standard deviation (RSD) of < 6% for resolution and < 10% for retention volume. The same sample and running conditions as for the column lot-to-lot comparison was used here.
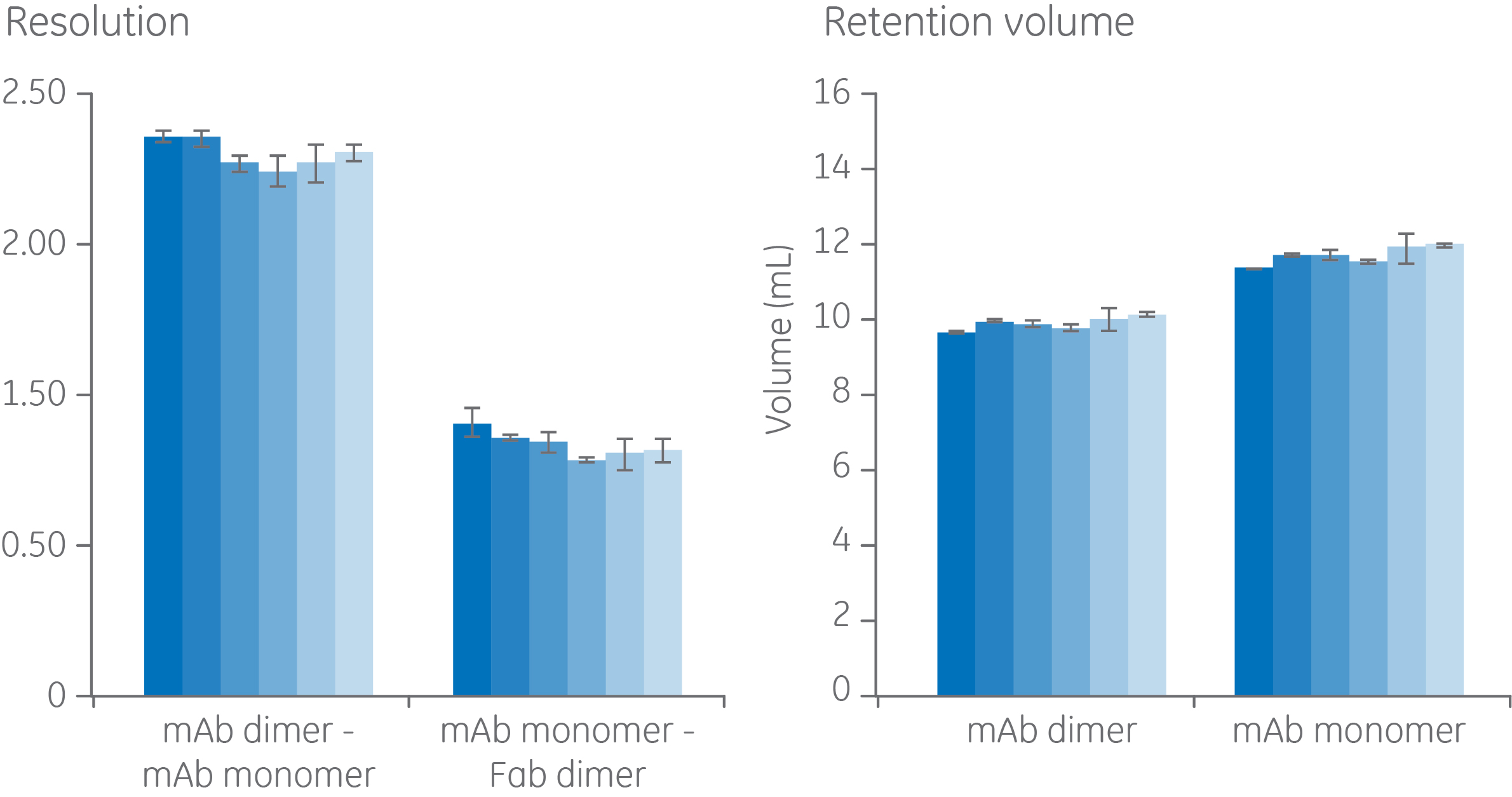
Fig 5. Comparison of six different Superdex 200 Increase batches of resin on resolution and retention volume in the purification of mAb and mAb aggregates. Same sample and running conditions as for Figure 5.
Change control notifications (CCN) ensures you that you are notified about changes that could potentially impact your product or process. The CCN service is available for free subscription.
We offer CCN for the following SEC columns:
Superdex 30 Increase 10/300 GL (29219757)
Superdex 75 Increase 10/300 GL (29148721)
Superdex 200 Increase 10/300 GL (28990944)
Superose 6 Increase 10/300 GL (29091596)
Each time a change in specifications is made to any of these columns, we let subscribers know by email.
For quality control purposes, we strongly recommend the 10/300 GL column format.
Learn more from the white paper Protein analysis with size exclusion chromatography