Are pure proteins a prerequisite for moving ahead with your research? Let me show how you can simplify your way to reaching this goal with the following example.
Easy transition to affordable automated purification
We expressed a histidine-tagged fusion protein that resulted in the formation of inclusion bodies. We solubilized these with urea. Using a manual purification workflow, we only achieved a purity of between 60% and 80%, even after diluting the sample ten times to start the refolding process. However, sample dilution resulted in handling large sample volumes, which proved to be cumbersome. Therefore, we evaluated an automated purification approach.
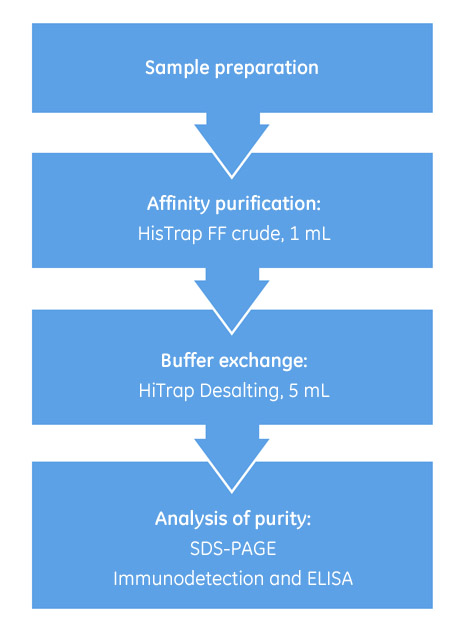
Our set-up included an ÄKTA start system, installed with UNICORN start control software, prepacked columns, and a fraction collector. Fig 1 illustrates the purification workflow.
Fig 1. Workflow illustrating the steps involved in purifying and refolding histidine-tagged proteins from inclusion bodies.
One-time method creation
The UNICORN start software enabled quick creation of the purification method. We used a HisTrap FF crude, 1 mL column, which was selected from the column list; parameters were adjusted as needed. The created method was also available for immediate use in future runs.
System pump for easy loading
We used the system pump to load the sample. This approach simplified the handling of large sample volumes and decreased hands-on time.
Automated collection of fractions
The fractions were collected using an automated fraction collector, and we could easily identify the peak fractions in the UNICORN start Evaluation module.
Straightforward buffer exchange
Imidazole, used in the elution buffer, was quickly removed from the pooled peak fractions using a HiTrap Desalting column. Compared with conventional dialysis, the desalting step greatly reduced process time.
A simplified workflow with improved purity
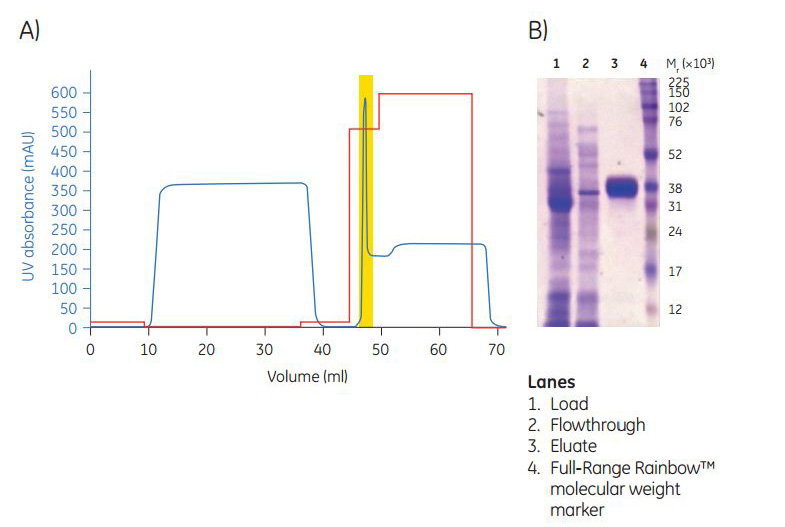
Fig 2A, the his-tagged protein was eluted in a sharp peak. We analyzed the pooled peak fractions using SDS-PAGE electrophoresis (Fig 2B).
Fig 2. A) Chromatogram showing the elution profile of N-terminal histidine-tagged protein on the HisTrap FF crude 1 mL column. Area highlighted (yellow) represents the pooled fractions. The high UV signal after the peak is due to the absorbance of imidazole at 280 nm.B) 12.5% SDS-PAGE profile of the protein fractions.
Compared with a manual workflow, an automated procedure minimizes handling and offers improved method parameter control. Also, prepacked columns provide a more consistent column performance than manually packed columns. Our result show that the automated purification method gave excellent separation of the target protein from contaminating cellular proteins. By using ÄKTA start, together with the prepacked affinity and desalting columns, the purity could be increased up to 1.6-fold compared with a manual approach (from 60% to 95%).
Read more about how automation can simplify purification of his-tagged proteins, allowing you to spend your time on what matters—your research.