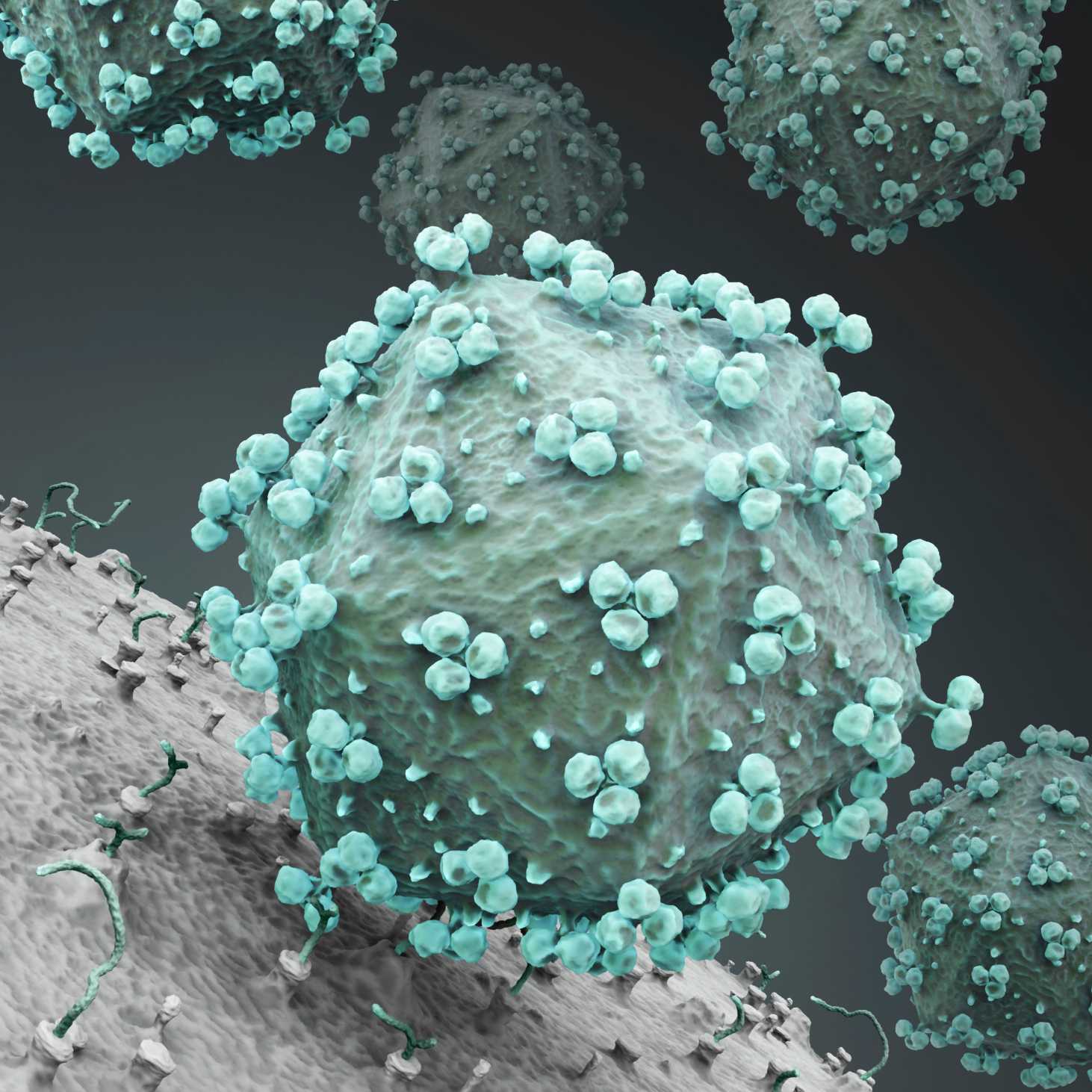
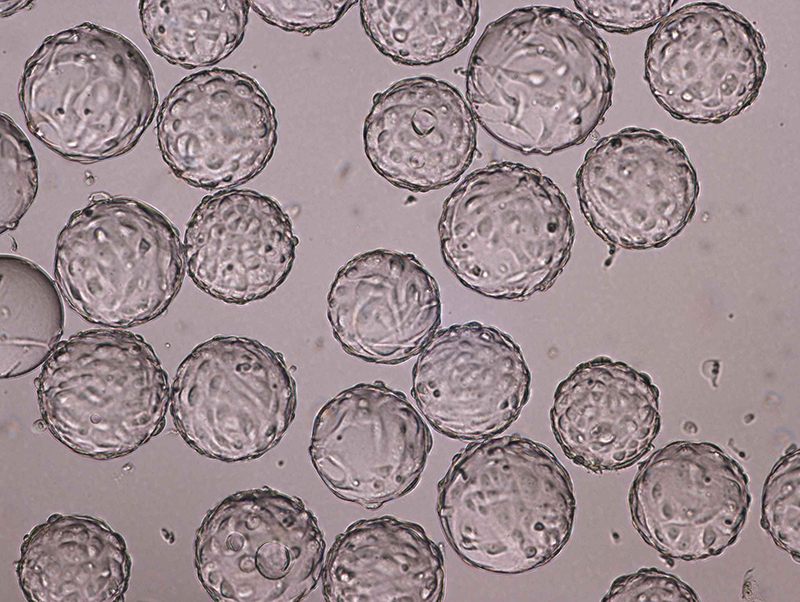
Vaccine manufacturing is complicated. At the same time, access to safe and efficient vaccines is crucial for global health. Here’s how training can help you tackle the challenges of vaccine development head-on.
The vaccine market is growing rapidly (1). But vaccine development and production are some of the most challenging tasks in the biopharma industry. Today’s vaccines are based on increasingly complex structures with specific targeted efficacy. Manufacturers need to focus on a quality-based approach for purification and viral safety.
Vaccine R&D projects are technically complex and inflexible, costly methods often drive the production processes. In addition, meeting international quality standards can be a real challenge. For these reasons, these molecules require advanced cultivation and purification strategies.
As for all biopharmaceuticals, vaccine manufacturing requires fast method development and reliable scale-up to a cGMP-compliant vaccine manufacturing. With such demanding molecules, this goal might seem daunting. However, training in vaccine development is one way to make sure the right skillsets are available to reach the goal.
Vaccine development training
To strengthen vaccine process development skills in the industry, GE’s Fast Trak team held a workshop in Shanghai, China last year. R&D scientists, process engineers, and manufacturing technicians from various vaccine industries in China and its neighboring countries joined in.
We held the workshop to support The Developing Countries Vaccine Manufactures Network (DCVMN) in its goal to help vaccine manufacturers in developing countries meet their emerging vaccine needs. The network is an international alliance of manufacturers, established in year 2000.
Today, DCVMN includes 50 vaccine manufacturers, in 17 countries and territories, producing and supplying over 40 different types of vaccines. Their vision is to protect all people against known and emerging infectious diseases, with the mission of increasing the quality and availability of vaccines affordable to all.
Tangible skills through hands-on vaccine process training
To really strengthen the skills and get hands-on process experiences, the participants spent half of the time in the lab. They cultured Vero cells on microcarriers using serum-free medium. And they performed bead-to-bead transfer of cells on microcarriers as well as scaled up the culture using single-use bioreactors. They also used various chromatography systems and column packing techniques.
The rest of the time was spent on lectures, which provided knowledge in important topics like upstream and downstream process development techniques, quality by design (QbD) best practices, and vaccine process economy considerations.
This training approach, together with the friendly and candid discussions among the trainers and participants, boosted the participants vaccine development competence and hands-on experience. Why not check out what Fast Trak courses are available for you?
References
1. Vaccine Market Analysis By Type (Live Attenuated Vaccines, Inactivated Vaccines, Subunit Vaccines, Toxoid Vaccines, Conjugate Vaccines, DNA Vaccines), By Application (Infectious Diseases, Cancer, Autism, Allergy) And Segment Forecasts To 2024, Grand View Research (2016)