By Kimberly Curtis, Kim Rawlins, Zhou Jiang, and Peggy Lio
Introduction
A platform approach has been increasingly adopted in bioprocess development to expedite project timeline and accelerate speed-to-market. Platform cell culture media, a key element of a platform bioprocess, is commonly developed using multiple representative clones aiming to produce optimal and balanced outcomes across all clones. Platform media leverages the similarities among molecules and offers advantages of cost and time savings associated with media screen and optimization. However, platform media may not achieve the maximum performance of individual clones. To better understand this limitation of platform media and facilitate decision making of media strategy, we assessed the quantitative difference in performance between platform media and clone-specific optimal media developed using in-house model CHO clones.
Experimental approach
Simulated cell culture media development
Platform and clone-specific media development were performed separately using three model CHO clones (Fig 1). An initial screen of six base media (ActiPro™ medium, NP1, NP2, NP3, NP4, and NP5) was performed using the three CHO clones in simple fed-batch culture. The top three base media exhibiting the highest VCD and titer across all three clones were selected for constructing ten prototypes following media mixture DoE design. The ten prototypes were evaluated with the three clones for cell growth, productivity, and metabolites during simple fed-batch culture.
Identification of optimal platform and clone-specific media compositions
The VCD and titer results from the base media screen were used to generate mixture profile plots that predicted the optimal platform and clone-specific media compositions. Briefly, a mixture DoE model was evaluated with a standard least squares fit, where titer was weighted at 0.8 and VCD was weighted at 0.2. Titer and VCD prediction formulas were used to create ternary plots that displayed the most desirable medium composition for the platform and clone-specific conditions. The theoretical performance of the platform versus clone-specific media compositions was confirmed experimentally with the three clones using simple fed-batch culture.
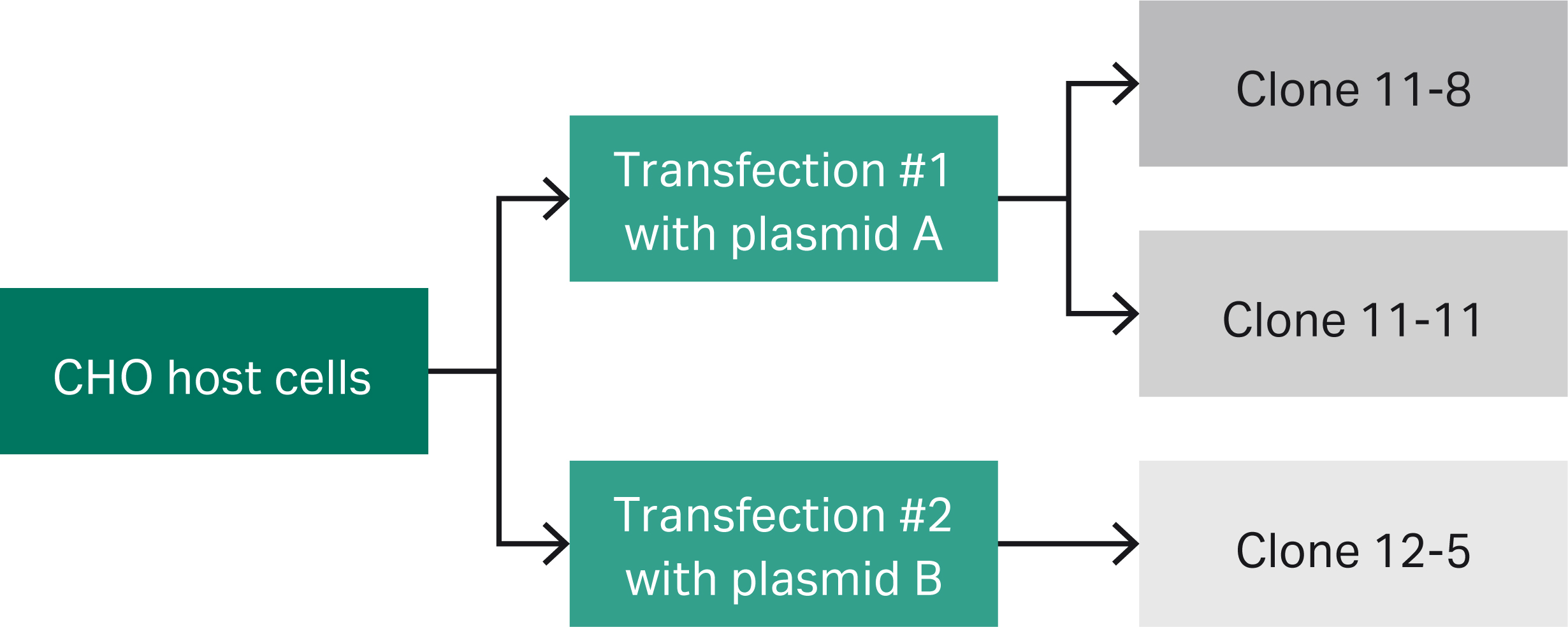
Fig 1. Lineage of model CHO clones.
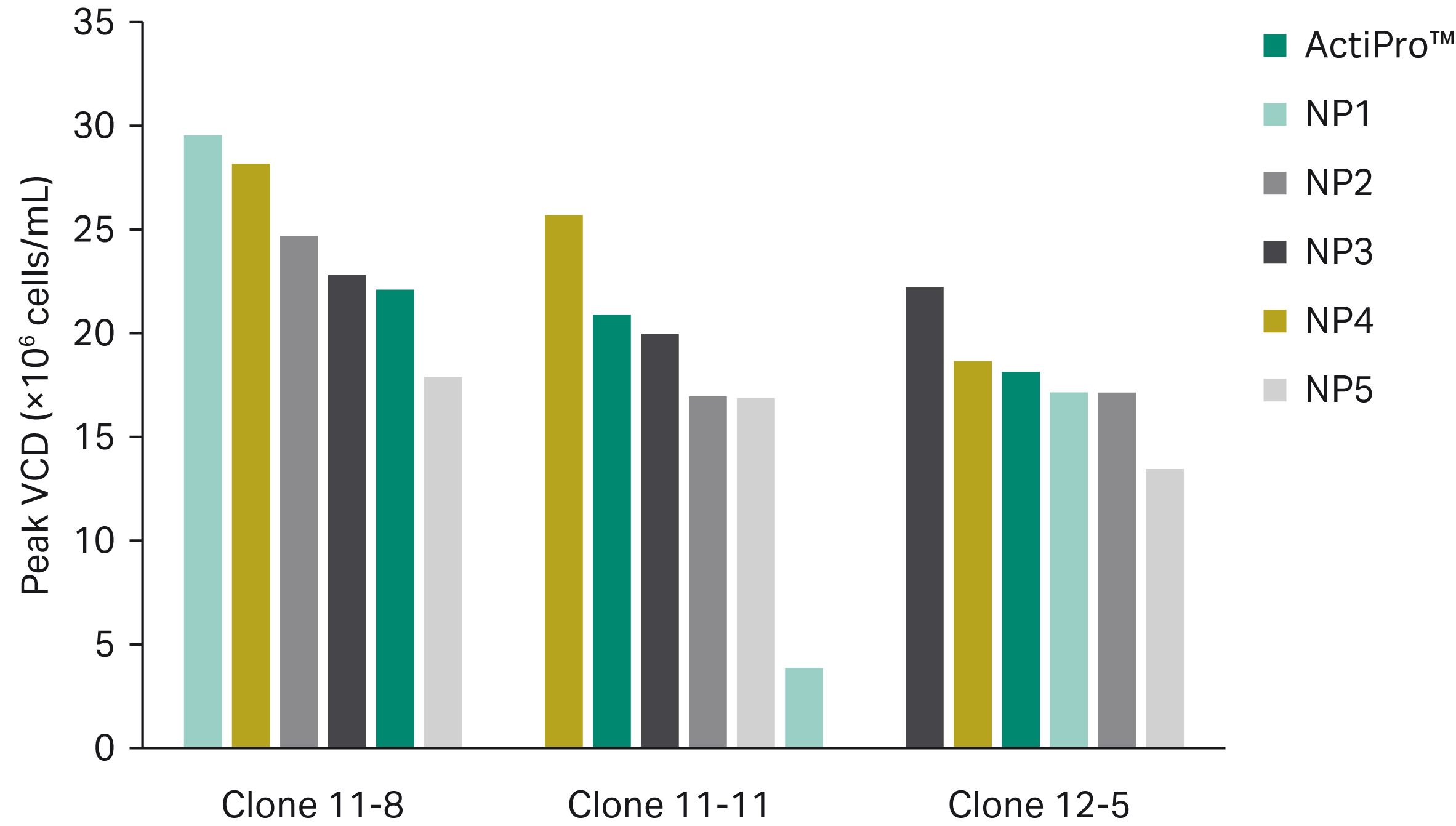
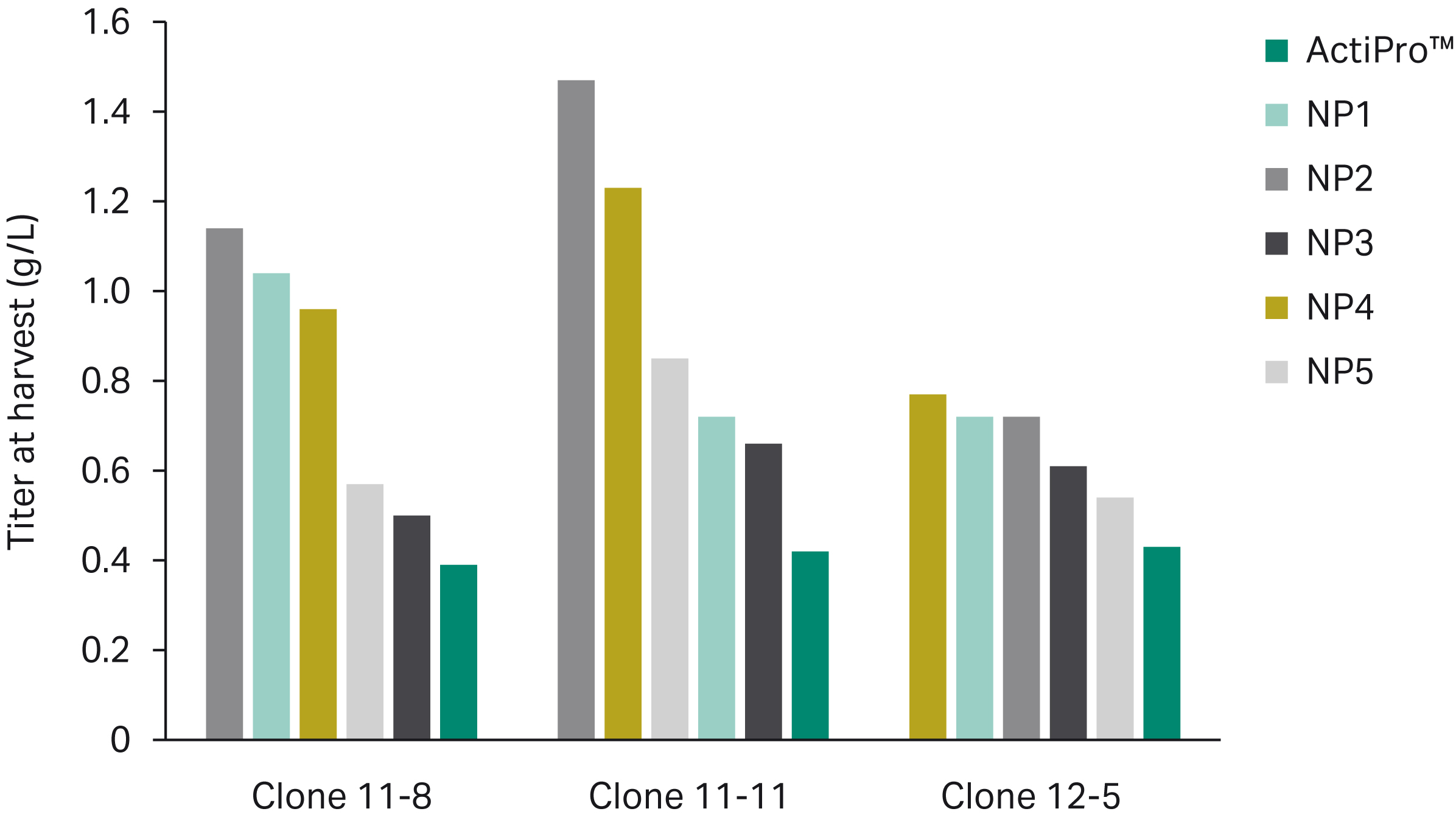
CHO clones exhibit different VCD and titer with each base media prototype
An initial base media screen demonstrated that the peak viable cell density (VCD) and titer achieved by each clone were attributed to different prototype media, illustrating how cell performance can vary among clones due to media composition. The peak VCD for clones 11-8, 11-11, and 12-5 was reached using base media NP1, NP4, and NP3, respectively (Fig 2A). Base media NP2 resulted in peak titer for clones 11-8 and 11-11; however, clone 12-5 attained peak titer with NP4 (Fig 2B). The top three base media prototypes across all clones (NP1, NP2, and NP4) were further evaluated in mixture combinations (see below).
(A)
(B)
Fig 2. Ranking of base media prototypes for peak VCD (A) and peak titer (B) across CHO clones 11-8, 11-11, and 12-5. The top three media across all clones (NP1, NP2, and NP4) were further optimized in a mixture DoE.
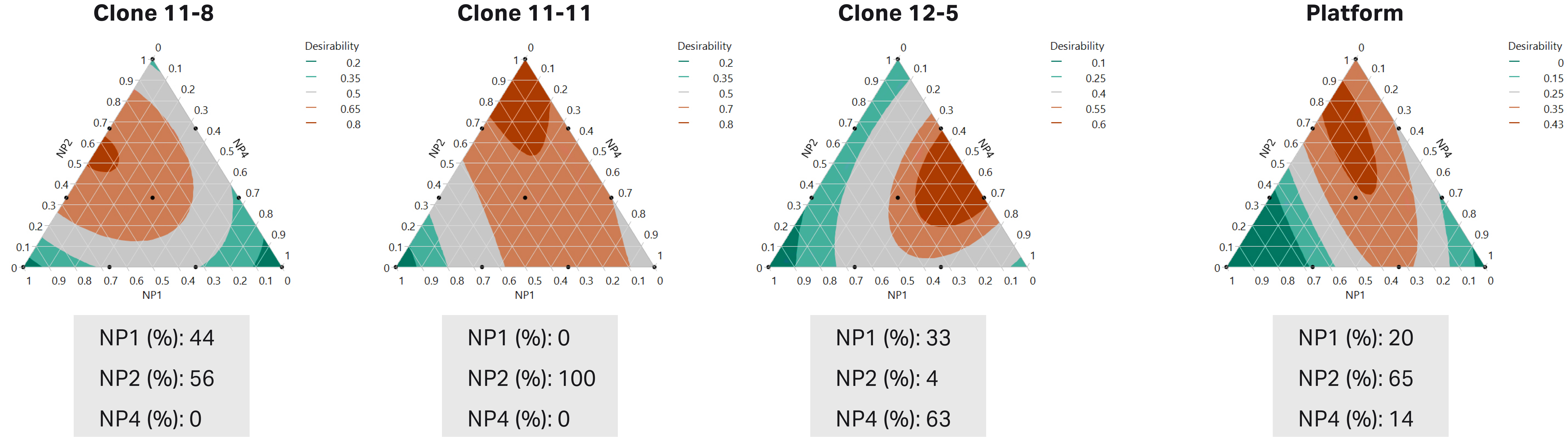
Mixture DoE identifies optimal platform and clone-specific media composition
Ten base media combinations of NP1, NP2, and NP4 were determined via mixture DoE and evaluated for performance using the three CHO clones in simple fed-batch culture. The VCD and titer results were used to obtain statistical predictions of cell performance using any combination of NP1, NP2, and NP4. The predicted desirability, which was weighted to primarily account for highest titer, of these prototype combinations is displayed with ternary plots (Fig 3). Each clone was predicted to achieve its highest titer using unique, clone-specific base media compositions (Fig 3A). For example, clone 11-8 was predicted to reach its highest peak titer using base media comprised of 44% NP1 and 56% NP2, while clone 11-11 would perform best using pure NP2. A base media comprised of 33% NP1, 4% NP2, and 63% NP4 was predicted to obtain the highest titer for clone 12-5.
The platform media composition was predicted to have balanced outcomes across all three clones and is comprised of 20% NP1, 65% NP2, and 14% NP4 (Fig 3B). Platform base media was predicted to compromise an average of 10% of titer across clones compared to the clone-specific base media.
(A)
(B)
Fig 3. Predicted optimal clone-specific (A) and platform (B) base media compositions based on maximum desirability from mixture DoE. On average across the clones, the titer achieved by the platform base media condition is predicted to be 10% less than the titer from the clone-specific conditions.
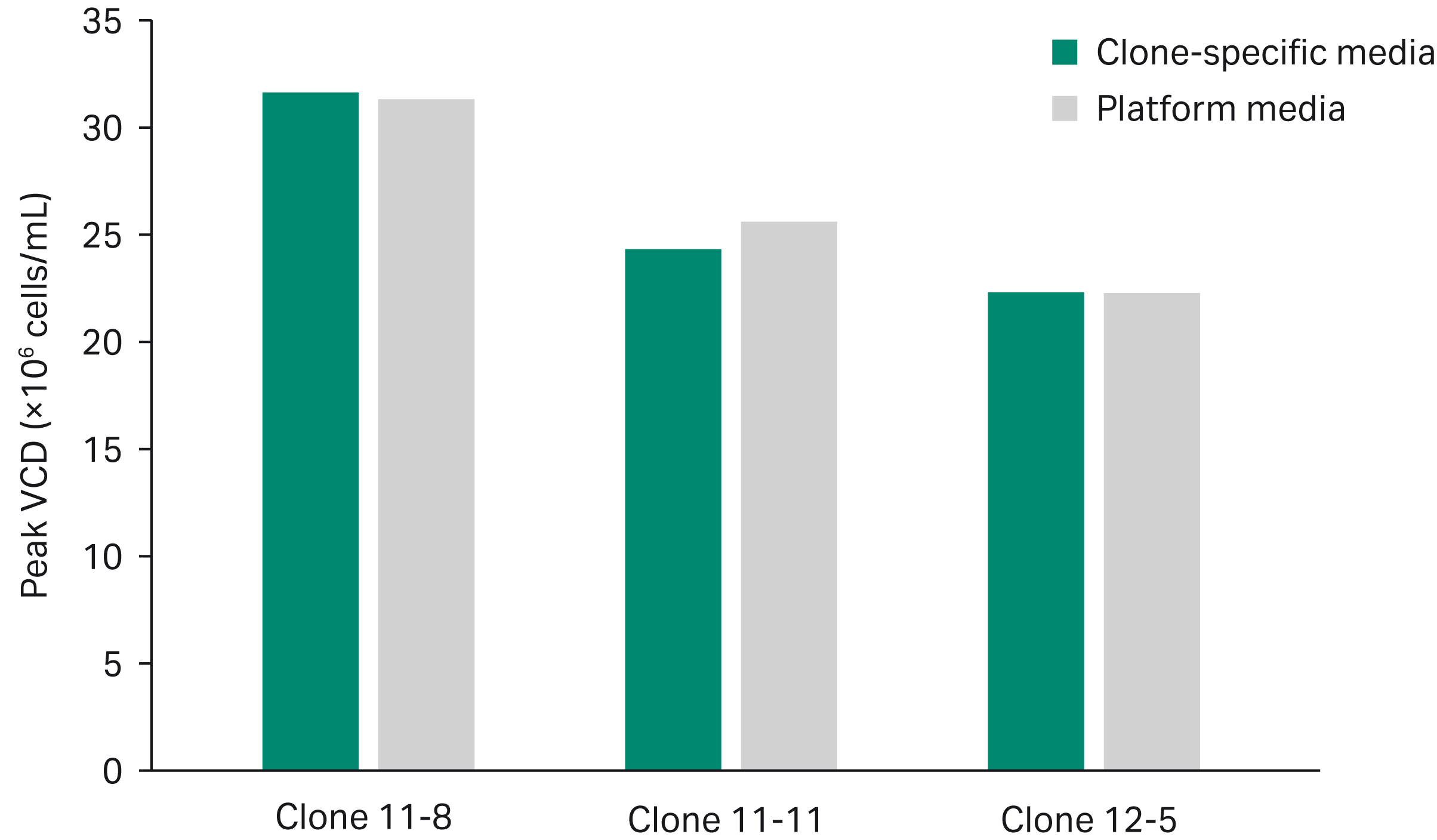
Experimental comparison shows a 10% compromise in titer when using platform vs. clone-specific media
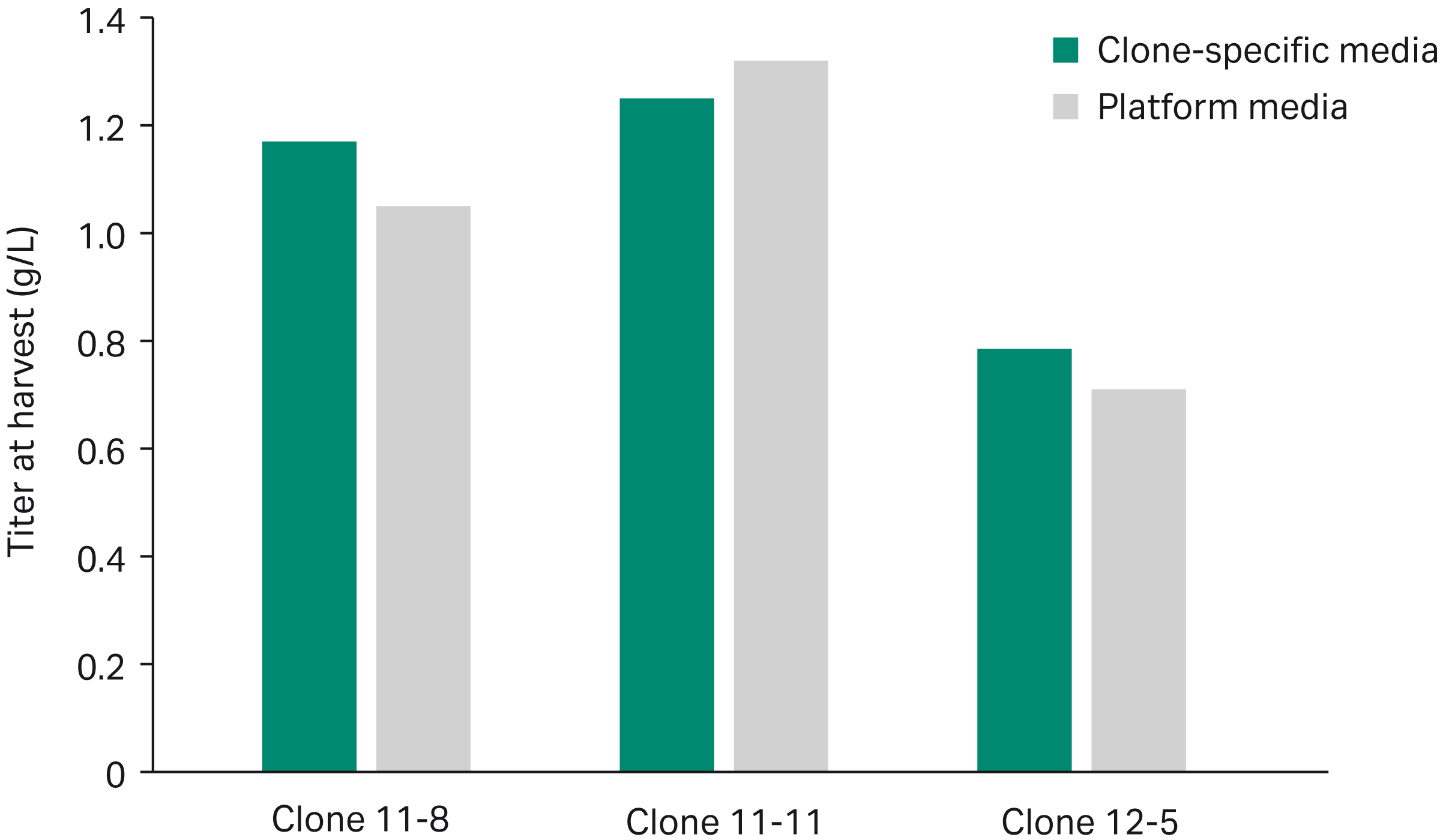
The optimal clone-specific and platform media compositions predicted to yield the highest desirability in the mixture DoE model were evaluated experimentally. The three clones were cultured in their respective clone-specific media as well as the overall most desirable platform media composition in simple fed-batch mode and monitored for growth and titer. Peak VCD was comparable between clone-specific and platform media across all clones, with a slightly higher VCD in clone 11-11 using platform media (Fig 4A). Clone-specific media exhibited a 10% increase in peak titer on average in clones 11-8 and 12-5 (Fig 4B), which was close to differences predicted by the model. Although platform media exhibited a 5% increase in titer compared to the clone-specific media for clone 11-11 (Fig 4B), both media compositions fell within the highest predicted desirability for that clone (Fig 3).
(A)
(B)
Fig 4. Experimental comparison of clone-specific versus platform media show differences in peak VCD (A) and peak titer (B) across three CHO clones. Titer was compromised using platform medium in two out of the three clones, varying as much as 10% between platform and clone-specific media.
Conclusions
- The base media providing the highest VCD and titer differed between CHO clones, regardless of whether the clones were derived from the same transfection event.
- Mixture DoE models predicted unique base medium compositions to yield the highest desirability for each clone. The platform medium was predicted to compromise titer an average of 10% compared to clone-specific media.
- Experimental comparison showed a 10% increase on average in titer using clone-specific media in two out of the three clones examined (Table 1). Clone 11-11 exhibited no benefit using clone-specific medium versus platform medium due to the overlap in media compositions that would yield high desirability
| Predicted (% change in titer) |
Actual (% change in titer) |
|
|---|---|---|
| Clone 11-8 | 7.14% | 10.26% |
| Clone 11-11 | 0.00% | -5.30% |
| Clone 12-5 | 12.50% | 9.55% |
Table 1. Comparison of the model predicted and experimentally determined percent change in titer using clone-specific vs. platform base media across three CHO clones.
Learn about cell line development services using platform cell culture media