By Emma Lind, Global Product Manager at Cytiva
You might be one of the many process developers who are working on a diversity of molecules such as enzymes, peptides, scaffolds, and other recombinant proteins. This constantly evolving molecular diversity brings challenges in chromatography process development. In this article, we focus on the challenges in purifying recombinant proteins that lack affinity chromatography solutions.
We asked about your challenges, and you told us
We asked 45 process developers about the challenges they have purifying recombinant proteins that cannot be purified with affinity chromatography. The top three challenges most process developers experience are:
- Low purity in the first step
- Slow process development time
- Lengthy, multistep purification processes
Indeed, in contrast to traditional monoclonal antibodies (mAbs), which have well-established processing protocols, the newly evolving molecules often do not have set purification platforms. As a result, you end up having to develop a new purification method for each protein, as explained by Emma Lind in this video.
Fig 1. Comments from process developers who can’t use affinity chromatography as a first capture step for recombinant proteins.
Lack of an affinity solution can result in low purity after the capture step
Benefits of affinity capture steps
Process developers often use an affinity chromatography step as their first protein purification step, or “capture step.” Affinity chromatography purification is favored because it is easy to implement and can give high purity in one step. If you have high purity after the capture step, you might need fewer chromatography polishing steps to get the purity you need. And fewer polishing steps mean higher yields and savings in time and cost during both process development and future manufacturing.
For example, in mAb production, protein A affinity chromatography is widely used for the capture step. Purity greater than 95% can be achieved after this step, which means that fewer steps are required before the final formulation steps.
There is no common affinity solution for all recombinant proteins
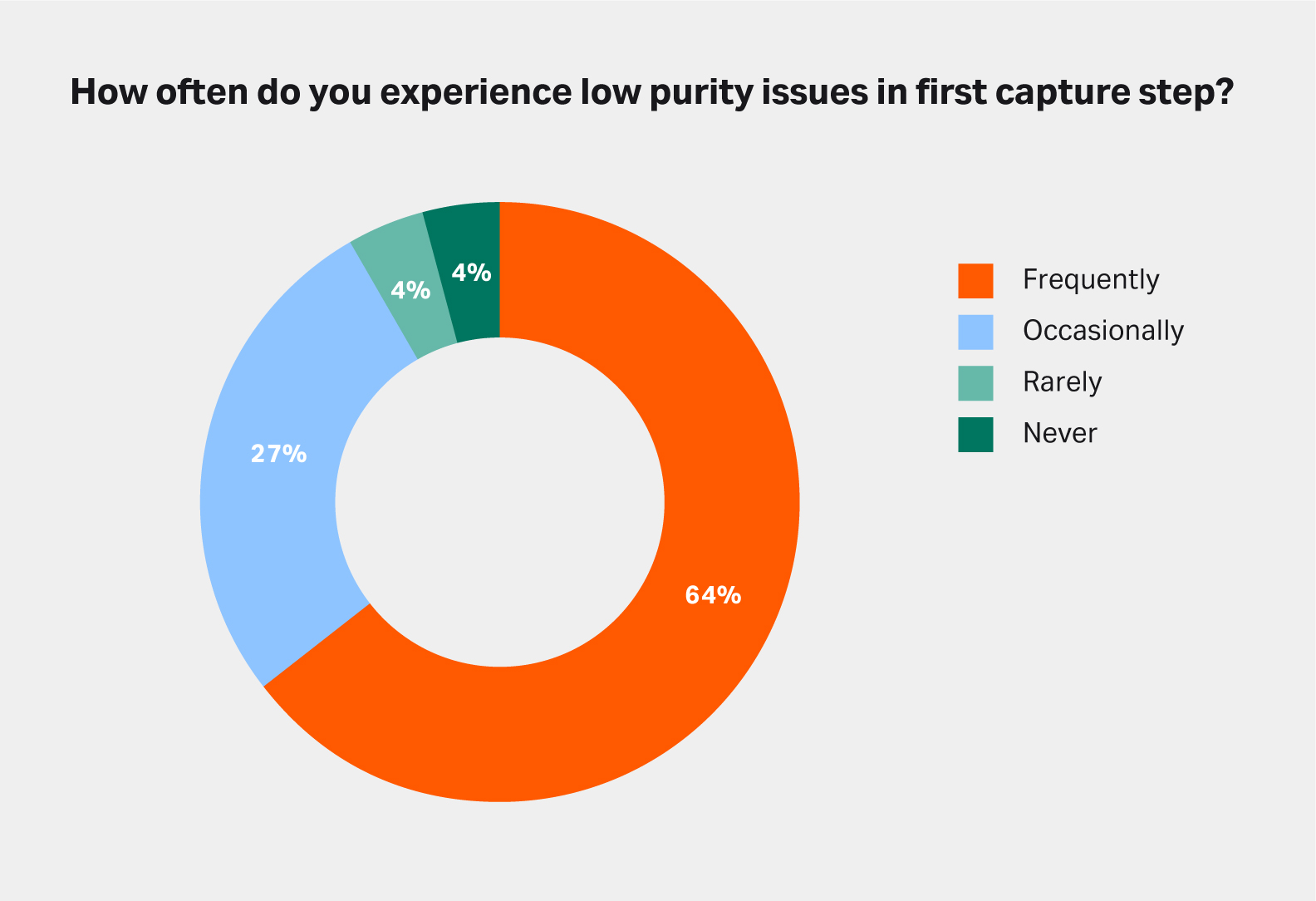
Many recombinant proteins do not have an existing affinity binding partner. Figure 2 shows that 64% of the survey respondents purifying non-mAbs frequently face problems with low purity in their first chromatography step. Also, when asked, more than 40% say that this low purity is a major issue in their work.
Therefore, these molecules need more chromatography steps to reach the needed purity.
Fig 2. Low purity in the first chromatography capture step is one of the main challenges faced by process developers working with a recombinant protein that cannot be purified with affinity chromatography. Source: Survey performed in December 2021. Sample: 45.
Developing a new purification process for each new molecule takes time
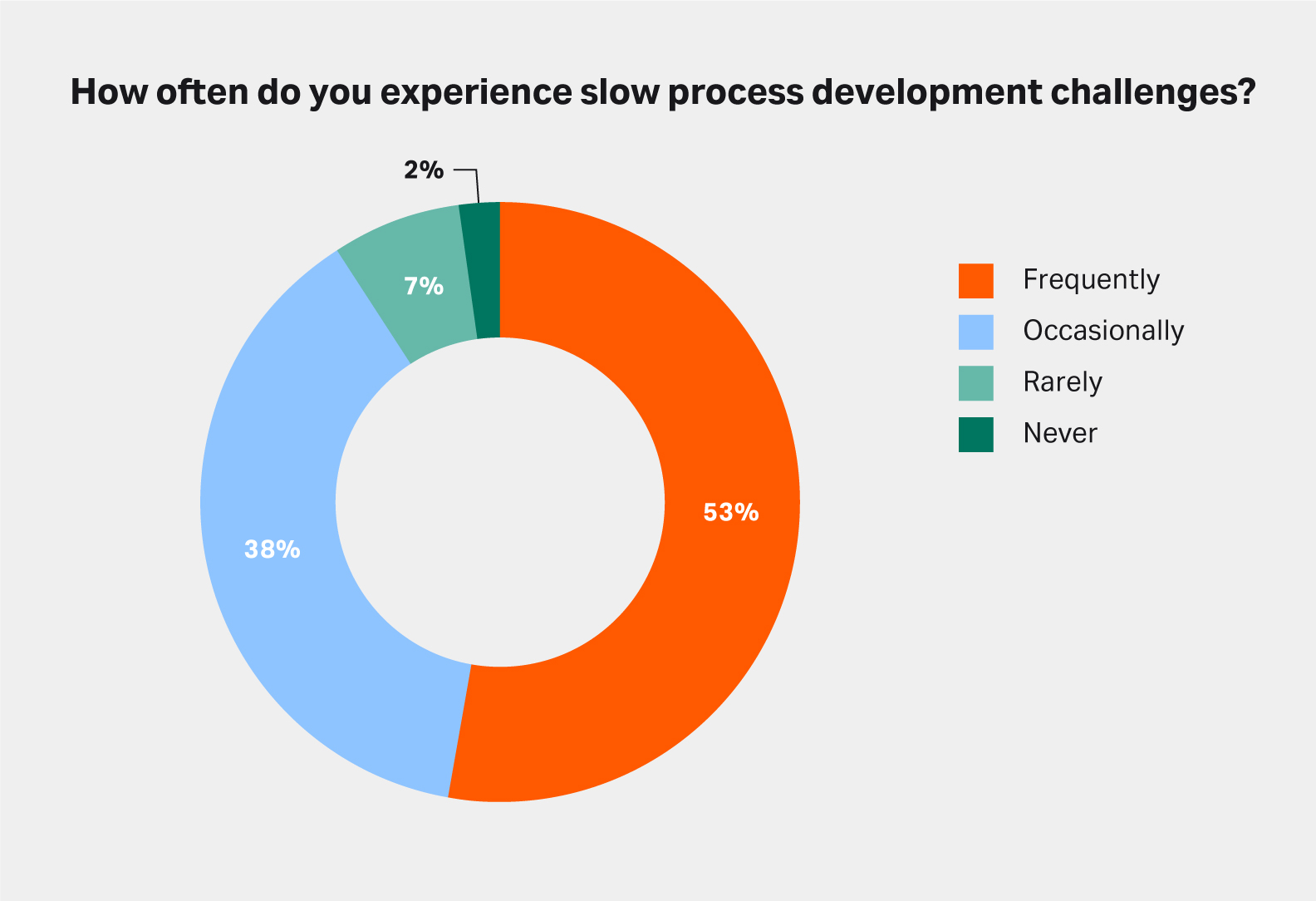
Figure 3 shows that 53% of process developers in the survey frequently face slow process development challenges with recombinant proteins. Indeed, when a molecule cannot be purified with affinity chromatography, you need to develop a complex, multistep purification process for it because each new molecule behaves differently during chromatography.
To reach the required purity and yield, your purification process might require a combination of many steps, such as ion exchange chromatography, hydrophobic interaction chromatography, and multimodal chromatography. Optimizing these methods all take time.
The whole screening and optimization process might take several months, and you risk not meeting time constraints. The risk of having a long process development phase can cause you to abandon the molecule. You may even abandon it before it’s up for consideration to begin the process development process.
Fig 3. Slow process development is another challenge faced when working with a recombinant protein that cannot be purified with affinity chromatography. Source: Survey performed in December 2022. Sample: 45.
Long chromatography processes generate increased cost
Almost half (43%) of survey respondents think that their chromatography process takes too long. When purifying a protein that cannot use affinity chromatography, you risk needing more chromatography steps in the process. A long chromatography process becomes a major contributor to your overall production time and costs and needs to be taken into account during early process development.
Coming soon! An affinity chromatography resin for purification of any recombinant protein
We heard you! Our research and development scientists developed Cytiva™ Protein Select™ resin that will allow you to use standard affinity chromatography to purify any protein that does not have an affinity binding partner.
Using Cytiva™ Protein Select™ resin will make your process development:
- Easier
- Faster
- More efficient