Single-use systems (SUS) in biopharmaceutical manufacturing give advantages such as improved flexibility and plant efficiency, but also raise concern for compounds leaching from the polymeric components and entering the process stream.
In a previous article, we presented how a biopharmaceutical leachables risk assessment can be performed over a whole bioprocess. We concluded that all consumables in the process, ending in bulk fill, were associated with low/medium risk, and the safety risk was deemed acceptable. This follow-up experimental study verifies the outcome of our initial assessment. Here, we mapped process equipment-related leachables (PERLs) over a complete single-use mAb process at pilot scale. Samples for analysis of leachables were taken at eight different steps over the process—from the bioreactor over the purification steps to the final bulk fill bag. The samples were analyzed with broad scope chromatography and mass spectrometry screening methods.
Our main findings were:
- PERLs present in the final bulk fill bag were different from the PERLs present in the upstream and early downstream steps. This finding concludes that leachables from early process steps, that is, before formulation, run a low risk of persisting through the process.
- Purified mAb in the bulk fill bags from two different capture steps and separate process trains showed the same low PERL impurity profile, unaffected by the different technologies used for the capture step. We performed a drug product-specific safety assessment on the results to exemplify how the low impurity levels were below safety concerns.
Introduction
Single-use systems (SUS) in biopharmaceutical manufacturing improve flexibility and plant efficiency but compounds leaching from the polymeric components and entering the process stream are still a concern. Such leachable compounds could negatively affect product quality, safety, and/or process performance.
The risk with leachables may be evaluated according to industry best practices guidance (ref 1). In an earlier study, we assessed a complete single-use generic manufacturing process for a monoclonal antibody (mAb)—from a 2000 L bioreactor to the final bulk fill bag. We started the evaluation with an extractables and leachables propensity assessment based on where in the process and under what conditions the consumable is used. The closer to the finished drug product, the higher the risk. Also, process conditions such as temperature, contact time, type of process fluid, and contact surface area affect the risk.
Certain process steps are expected to reduce leachables to a large extent, so applying risk mitigation considerations changed the risk classification of all consumables in upstream and purification steps to low. Consumables used in the final steps remained as medium risk.
Extractables data were then collected for the consumables in the final steps, as is required for medium- and high-risk applications. The data on estimated accumulated extractables from these steps was used for a chemical safety assessment. The conclusion from the assessment was that safety risk was acceptable and no leachables studies would be required before fill and finish.
This follow-up experimental study with analysis of leachables over a complete single-use mAb process at pilot scale provides greater clarity around the following:
- Is the leachables risk low with consumables used in the upstream cell culture and the downstream purification steps, as was considered in the extractables assessment?
- Will extractables data allow prediction of actual leachables from consumables used in a process?
- Is the safety risk of the leachables present with the purified mAb in a bulk fill bag acceptable?
- Is the leachables profile affected by the capture step, whether the MabSelect PrismA™ resin prepacked in the ReadyToProcess™ column or the Fibro™ PrismA unit was used?
Closed connected single-use mAb process
The closed connected single-use mAb process was performed at pilot scale with 50 L perfusion culture, cultivated over 10 days (d) after reaching cell density equilibrium. The purification process was divided into two separate processing trains. One process used a MabSelect PrismA™ prepacked ReadyToProcess™ column for the capture step. The second process used a pilot-scale Fibro™ PrismA unit, a fiber-based technology, which enables single-use rapid cycling chromatography. You can find out more about the process here.
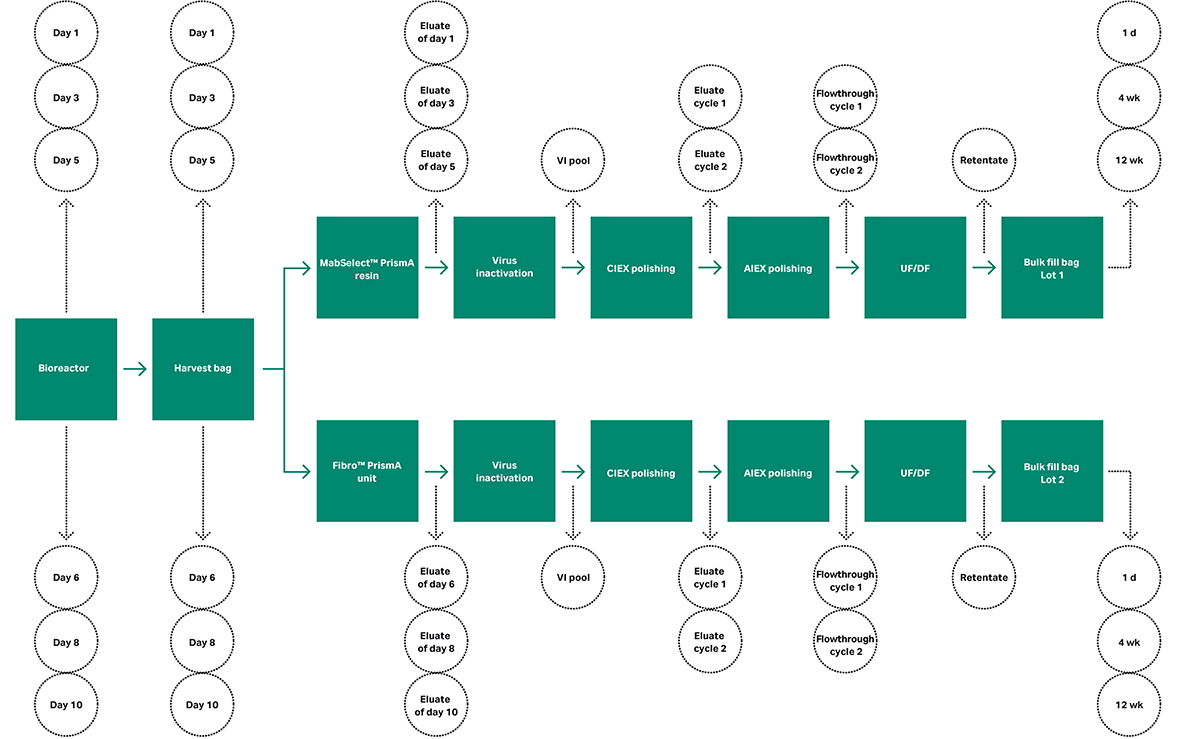
We collected 36 samples at different sampling points, illustrated in Figure 1. Samples were withdrawn from the bioreactor on days 1, 3, 5, 6, 8, 10, and after harvesting on the same days. Corresponding samples were collected after the capture steps on MabSelect PrismA™ resin and Fibro™ PrismA unit.
Next, samples were taken after virus inactivation (VI), followed by samples after polishing with the Capto™ S ImpAct prepacked ReadyToProcess™ column (cation exchange chromatography [CIEX]) and a membrane adsorber Q (anion exchange chromatography [AIEX]), both performed in two cycles. Finally, samples were taken from the ultrafiltration/diafiltration (UF/DF) retentate and the sterile filtered material in the bulk fill bags after 24 h, 4 weeks (wk), and 12 wk refrigerated storage.
Fig 1. Samples (green circles) were withdrawn at different locations (green arrows) in the process with two separate purification trains.
Sample preparation
The sample preparation methods were aimed at a broad screening with the ability to detect a wide range of organic compounds. Recovery of expected PERLs of different classes of compounds was studied for the sample preparation methods to become as general as possible.
Two sample preparation methods were used, depending on the sample matrix. Liquid-liquid extraction (LLE) in ethyl acetate at acidic/alkaline pH was performed on samples from the bioreactor, harvest bag, and Capto™ S ImpAct CIEX eluate. Protein precipitation (PP) in cold acetonitrile was performed on the other samples. An additional LLE sample preparation was performed on samples from the final bulk fill bag to allow for better conditions for GC analysis.
We prepared test control samples with a composition similar to the sample matrices but without contact with the process consumables. The cell culture medium was used as the test control for samples in the upstream phase and the appropriate buffers, prepared from stock solutions using an in-line conditioning (IC) system, were used as test controls for samples in the downstream phase.
The test control for samples from the bulk fill bag was freshly prepared buffer in glass.
Analytical methods
Leachables were analyzed for volatile organic compounds with headspace gas chromatography-mass spectrometry (GC-MS). Semi-volatile organic compounds were analyzed with GC-MS. Nonvolatile organic compounds were analyzed with liquid chromatography (LC) high resolving MS (HRMS) performed with electrospray ionization (ESI) in positive and negative modes. All methods were untargeted screening methods previously established and qualified in our laboratory. All analyses were preceded by a system suitability test developed for each method and technique.
We identified detected compounds using databases. To increase confidence in the identity of a tentatively identified compound, we interpreted spectra MS/MS data when possible.
The concentration of a detected compound was estimated by a semiquantitative internal calibration method. Data analysis was in general performed using the test control as negative control and no strict threshold was applied.
Validation study
Qualification of the analytical methods for accuracy, precision, and recovery was performed for nine compounds predicted to be present in the final bulk fill bag, listed in Table 1. Samples from the bulk fill bag on day 1 were spiked at three concentrations within the ranges in Table 1.
We analyzed spiked samples together with non-spiked samples using the same sample preparation methods that were applied to the final bag sample, that is, protein precipitation and the additional LLE for GC-MS samples. Thereafter, we analyzed the samples using LC-MS/ESI in positive and negative modes and by GC-MS using the methods described above.
Table 1. Compounds used for qualification study
| Compound | Chemical Abstracts Service (CAS) registry number | Concentration range (µg/mL) | Method |
| 2,4-Di-tert-butylphenol | 96–76–4 | 0.002–0.02 | GC-MS |
| Hexaethylene glycol | 2615–15–8 | 0.002–0.02 | LC-MS ESI+ |
| 1-Methyl-2-pyrrolidone | 872–50–4 | 0.002–0.02 | LC-MS ESI+ |
| Bis(2,4-di-tert-butylphenol) phosphate, (bDtBPP) | 69284–93–1 | 0.002–0.02 0.04–0.4 |
LC-MS ESI+ |
| Caprolactam | 105–60–2 | 0.04–0.4 | LC-MS ESI+ |
| Hexanoic acid | 142–62–1 | 0.04–0.4 | LC-MS ESI- |
| Laurolactam | 947–04–6 | 0.04–0.4 | LC-MS ESI+ and LC-MS ESI- |
| Bisphenol A bis(2,3-dihydroxypropyl) ether, (BADGE.2H2O) | 5581–32–8 | 0.04–0.4 | LC-MS ESI+ and LC-MS ESI- |
| Palmitic acid | 57–10–3 | 0.04–0.4 0.5–1.5 |
LC-MS ESI- |
Triplicate samples showed precision (relative standard deviation, RSD) between 0.8% and 37%. The highest deviation was with the GC-MS samples prepared with LLE, as expected. Accuracy varied between 1.2% and 271% and was correlated to the concentration used for calculating the relative response factor. This is because the response is not linear over a wide concentration range.
Compounds could be detected at a relevant concentration (0.02 or 0.4 µg/mL) with recovery between 68% and 180% with at least one method. We could, however, only detect bDtBPP at concentrations above 1 µg/mL, and palmitic acid was not detected above the background level in samples with high mAb-concentration.
Results
Mapping of PERLs over the whole process
Certain process steps are expected to reduce PERLs to a large extent, that is, behave as sinks of leachables. To find out how this works for our mAb process, we compiled the results from all 36 samples analyzed with HS-GC-MS, GC-MS, and LC-MS for the purification process with MabSelect PrismA™ chromatography resin (lot 1) and the purification process with Fibro™ PrismA units in the capture step (lot 2).
Approximately 150 different compounds were reported with the analytical methods, showing the complexity of these types of samples. To focus on the PERLs and disregard compounds related to the cell growth process and the mAb, we used our database of more than 1100 identified extractable compounds from single-use components and reduced the data sets to compounds we had previously observed as extractables.
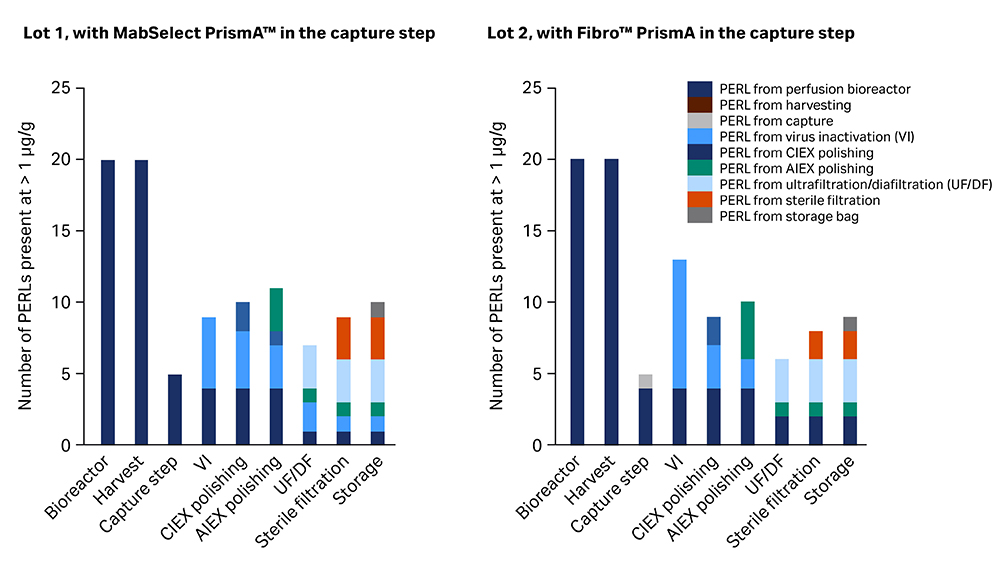
The concentrations of leachables were correlated to the mAb concentration of each sample. A PERL that was never present above 10 µg/g was regarded as non-significant for the study and was not included. The results on how many PERLs were present in the different process steps are shown for both lots in Figure 2, illustrating where in the process PERLs were removed and where new PERLs were added. Any individual result below 1 µg/g was excluded from the plots.
Fig 2. Number of PERLs present at > 1 µg/g mAb at each sampling point. Additions are marked in a new color for each step.
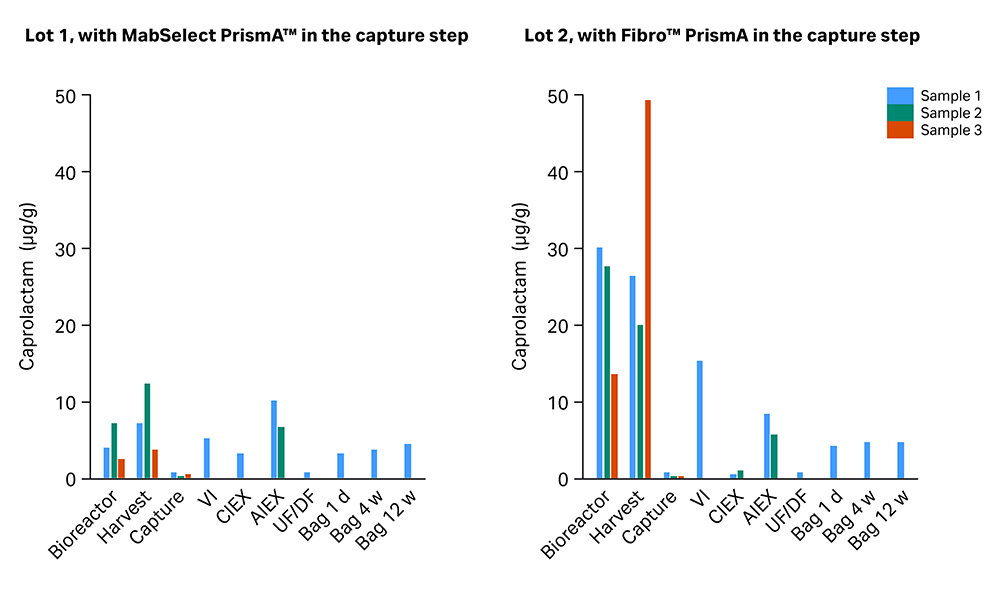
We noted that a few compounds are present throughout the end-to-end process. These are polymethylcyclosiloxanes and caprolactam, which are ubiquitous in single-use components and present in consumables for all process steps. The level of caprolactam measured in individual samples is shown in Figure 3, illustrating that both the capture step and the UF/DF formulation step are effective sinks for this PERL.
Fig 3. Level of caprolactam measured in all individual samples.
Leachables in the upstream process
Cell cultivation was performed using Xcellerex™ XDR 50 integrated with the Xcellerex™ Automated Perfusion System (APS) and was harvested for 10 d. Find out more about the perfusion cell culture process here. Samples were withdrawn for analysis of leachables from the bioreactor and the harvest bag (Fig 1).
The results from the analysis showed around 50 compounds for each of the samples of which 17 were predicted from the compilation of extractables data we had made for the consumables. Our attempts to predict any quantity of PERLs based on extractables data were, in most cases, far from precise because of the complexity of all sources of leachables from the many consumables in the process. Instead, extractables data were highly useful as a comprehensive list for PERLs and in correlating our findings from this leachables study. Compounds that were not predicted may be related to the cell growth process and were excluded unless they were present in our database for extractable compounds.
All PERLs present above 1 µg/g mAb in the bioreactor were also present in the harvesting bag, as illustrated in Figure 2. Thus, these were present in the load samples for the next step, which were the two alternative capture steps.
Downstream capture step – MabSelect PrismA™ resin vs Fibro™ PrismA unit
As expected, both capture steps based on protein A affinity binding efficiently removed leachables from the upstream step. The few PERLs analyzed in the eluates of MabSelect PrismA™ were polydimethylsiloxanes and caprolactam. The eluates of Fibro™ PrismA showed additional octanoic acid.
Virus inactivation (VI) step
Each daily eluted product pool was collected for VI in Cellbag™ reactor bags for WAVE™ bioreactors. The sub-batches were pooled for the separate processing trains after VI and leachables were analyzed in the two pools. The results showed that PERLs present in the eluates from the capture step was still present, as expected, with some additions of carboxylic acids and unidentified compounds.
Polishing steps
The eluates from the CIEX purification on Capto™ S ImpAct prepacked ReadyToProcess™ column and the flowthrough from the AIEX purification on membrane adsorber Q for each of the processing trains showed only a few reductions and additions of PERLs.
UF/DF formulation step
The purified material from polishing was concentrated and then buffer exchanged to the formulation buffer. The final retentate was then analyzed for leachables with results showing that several polydimethylsiloxanes, caprolactam, and antioxidant degradation products had been highly reduced in the UF/DF formulation step. Compounds related to bisphenol A bis(2,3-dihydroxypropyl) ether (BADGE.2H2O) had been added as predicted from extractables data for the tangential flow filter.
Sterile filtration and storage in bulk fill bags
The final retentate from UF/DF was filtered into storage bags using a pump and ULTA™ Pure HC capsule filter. The bulk fill bags were stored refrigerated, and samples were withdrawn and analyzed for leachables after 1 d, as well as 4 and 12 wk. Results reported at a level >1 µg/g mAb (ppm) are listed in Table 2 for the two lots.
Table 2. Results on PERLs present at >1 µg/g in bulk fill bags, semi-quantitative determination
| Result (µg/g) | |||||||
| Lot 1 (MabSelect PrismA™ resin) | Lot 2 (Fibro™ PrismA unit) | ||||||
| Compound | CAS number | 1 d | 4 wk | 12 wk | 1 d | 4 wk | 12 wk |
| Hexamethyltrisiloxane-1,5-diol | 3663-50-1 | 18 | 4 | 18 | 12 | 8 | 12 |
| Bicyclohexyl-dicarboxylic acid-related compound, Mw 254.152 | N/A | 5 | 6 | 9 | 6 | 9 | 9 |
| Caprolactam | 105-60-2 | 3 | 4 | 4 | 4 | 5 | 5 |
| Laurolactam | 947-04-6 | 1 | 2 | 2 | 1 | 1 | 1 |
| Hexamethylcyclotrisiloxane | 541-05-9 | 1 | 1 | 5 | 3 | 1 | 1 |
| 3,5-bis(1,1-dimethylethyl)-1-hydroxy-4-oxo-2,5-cyclohexadiene-1-propanoic acid | 83237-15-4 | 1 | 0 | 2 | 1 | 1 | 1 |
| Pentaethylene glycol | 4792-15-8 | 1 | 1 | 0 | 1 | 1 | 1 |
| Hexaethylene glycol | 2615-15-8 | 1 | 1 | 1 | 1 | 1 | 1 |
| BADGE.2H2O-related compound (C27H40O10 | N/A | 1 | 1 | 1 | 1 | 1 | 1 |
| Benzoic acid | 65-85-0 | 0 | 0 | 87 | 0 | 45 | 85 |
| Hexanoic acid | 142-62-1 | 0 | 0 | 8 | 0 | 0 | 8 |
The two highest reported compounds at day 1 in Table 2 are related to platinum-cured silicone and thermoplastic elastomer (TPE) tubing. Caprolactam, laurolactam, and polyethylene glycols originate from the normal flow filter used for sterile filtration. The compound related to BADGE.2H2O is from the tangential flow filter used in UF/DF. There is no difference between lot 1 and lot 2 that could be attributed to the different capture steps.
Additional samples were withdrawn after 4 and 12 wk storage. Two PERLs which were below detection level on day 1, were seen to increase during storage. Both compounds, benzoic acid, and hexanoic acid are correlated to the materials of the storage bag.
Chemical safety considerations
A drug product-specific safety assessment based on impurity level and dosing regimen may be conducted to evaluate patient safety aspects of leachable compounds. For our assessment, we assumed a daily dose for the patient as 10 mg/person/d, parenteral.
A safety concern threshold (SCT) (ref 2) was set as the threshold of toxicological concern (TTC) limit at 1.5 µg/person/d with treatments > 10 yr to a lifetime (3). This limit is associated with a negligible risk (theoretical excess cancer risk of < 1 in 100 000 over a lifetime of exposure) and can in general be used for most pharmaceuticals as a default to derive an acceptable limit for control.
The SCT and assumed daily dose can be used to calculate what impurity levels would cause exposure to patients below the threshold:
SCT (µg/d) = Impurity level (µg/g) × dose (g/d)
Impurity level (µg/g) = SCT (µg/d) / dose (g/d) = 1.5 (µg/d) / 0.010 (g/d) = 150 µg/g
We found that all PERLs in Table 2 were reported at a level below 150 µg/g, thus below the SCT. It implies that no further toxicological evaluation should be needed for the compounds, concerning the dosing regimen.
An analytical evaluation threshold (AET) can be calculated from the threshold set at 150 µg/g and the mAb concentration in the bag (35.8 g/L):
AET (µg/mL) = 150 (µg/g) × 35.8 (g/L) = 5370 (µg/L) = 5.37 (µg/mL)
The analytical methods must be able to detect leachables at the AET level to be fit for purpose. Our validation study showed that this is achieved with the compounds listed in Table 1 except palmitic acid, which was not detected above the background level. Palmitic acid was, however, predicted from worst-case extractables data to be present at < 4 µg/mL at 12 wk storage and not expected to pose risk to patient safety.
Conclusions
Our mapping of process equipment-related leachables (PERLs) over the different process steps of a pilot-scale mAb manufacturing process illustrated how PERLs were removed in purification steps while new PERLs were added from the single-use components used in the next step. Leachables from the upstream process were largely removed in the capture step and PERLs from the VI and polishing steps were highly reduced in the formulation step. Focus for a leachables assessment should therefore be placed on the final steps, that is, formulation, sterile filtration, and storage.
The results on PERLs in the bulk fill bags showed that the two purification process trains with different technologies for the capture step resulted in the same low impurity level and the same profiles in both cases. Our chemical safety assessment showed that the level of PERLs in the purified mAb is of no safety concern assuming the drug dosage described in our example.
This study confirms that a theoretical risk assessment based on extractables data from a supplier of single-use equipment gives you direction on where to focus your leachables testing. It also confirms that some process steps will act as leachables sinks, which is useful information when designing a process. The data presented describes a mAb process although the same approach could be applied for any other processes such as viral vectors or mRNA. Having a holistic view of the end-to-end process, consideration of leachables during the design phase, and using well-characterized consumables can speed up the route to regulatory approval.
- Sexton, AW, Rusu, AD, Smalley, C, Tapiawala, D, Low, D, Madsen, GL et al. Best practices guide for evaluating leachables risk from polymeric single-use systems used in biopharmaceutical manufacturing. BioPhorum Operations Group, June 26, 2018. Accessed April 8, 2022.
- Paskiet, D, Jenke, D, Ball, D, et al. The product quality research institute (PQRI) leachables and extractables working group initiative for parenteral and ophthalmic drug products (PODP). PDA J. Pharm. Sci. Tech. 2013;(67):430–447. doi: 10.5731/pdajpst.2013.00936
- International council for harmonisation of technical requirements for pharmaceuticals for human use (ICH), “Assessment and control of DNA reactive (mutagenic) impurities in pharmaceuticals to limit potential carcinogenic risk, M7 (R1)”, March 31, 2017, Accessed April 8, 2022.