Written by: www.themedicinemaker.com
In May 2019, Cytiva brought experts together at its Uppsala, Sweden, site for Bioprocess Days: an event to discuss the future of bioprocessing. One of the key themes was the role that digital technology, analytics and data will play. This article was developed based on an interview with Jun Huang (Director/Team Leader; Process Monitoring, Automation and Control at Pfizer) who presented a case study on the “Industrial internet of things” at the event.
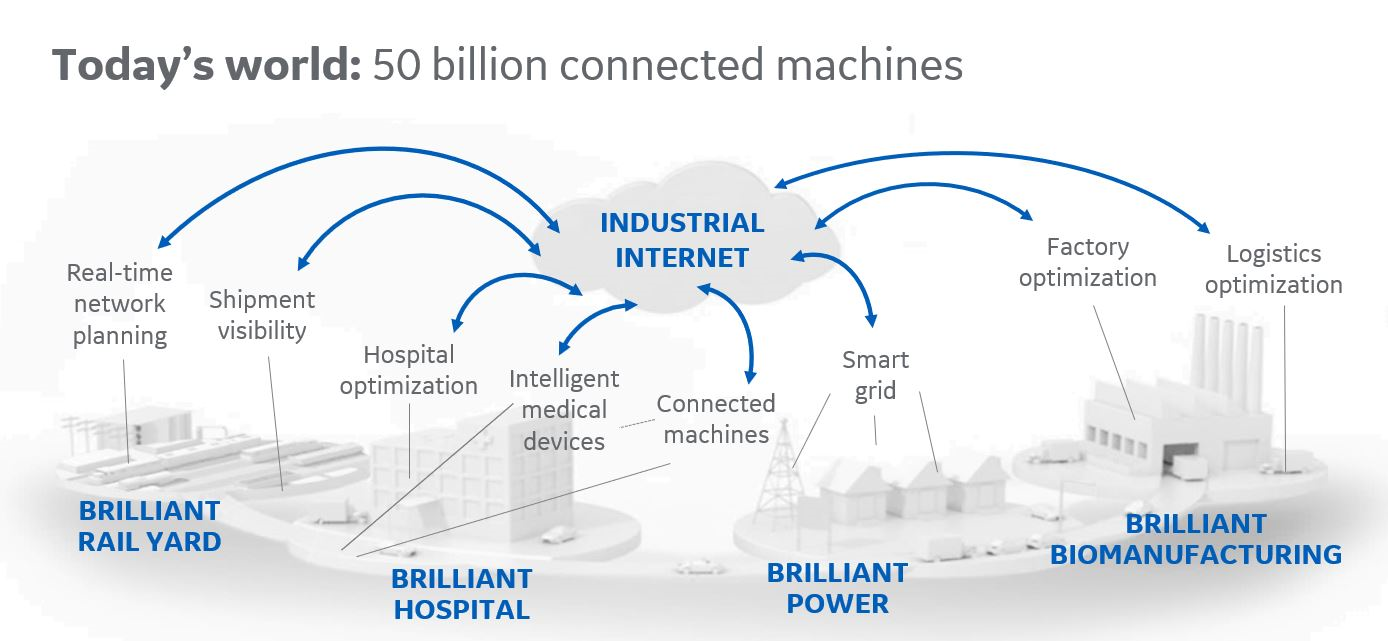
Digitalization means different things to different people. For me, it starts with connectivity and data. Pharmaceutical manufacturing generates a lot of data, and in many cases, operations are highly automated, but are they truly digitalized? I think many systems working in silos suggests they are not. For example, does your process control system talk to your quality system? Technologies like the industrial internet of things (IIoT) will release the data that is trapped in these silos by enabling connectivity between systems.
The idea is to combine and contextualize data from disparate sources and different core systems so that you can make it available to the people who need it; this could be an operator on the manufacturing floor, a plant supervisor or a senior manager. Your data would be unified in a central data hub and accessed by the people who need it.
This central data hub could be accessed through a persona dashboard – tailored to the needs of the individual decision-maker. For example, on the factory floor, people would be looking at operating the system in the best and most efficient way, so they might see some quite granular information. The plant manager, on the other hand, would care mainly about key performance indicators and higher-level metrics. And senior management will be keeping track of performance at the enterprise level, across various sites, enabling them to develop a long-term strategy for the entire network.
But a key question is, what exactly are these people supposed to do with all of this centralized data? Data is useless unless you can turn it into an intelligent decision. This is where analytics, AI and machine learning come in. IIoT enables connectivity, data gathering and contextualization, but you need analytics to tell you what to do with it and how to apply it to decisions regarding production.
At a process level, you can use IIoT in combination with advanced analytics in the existing process control system to improve process robustness and increase yield, ultimately enhancing productivity. In the quality and compliance department, the aim is to make sure the product is made within quality specifications. IIoT enables connectivity between quality and the production floor, allowing you to identify deviations quickly and make adjustments. Then at a higher level, real-time changes in demand could inform decisions about production.
“Turning data into intelligent decisions is the goal of digitalization, but to do that, companies must create the right culture.”
These decisions could be made by an operator or a manager, or, in some cases, an automated process control system. Imagine developing a model that, based on data generated by the IIoT, could manipulate your process so that an economic target or quality measure is met. The model might be able to go beyond real-time monitoring and decision making by predicting deviations or failures before they occur, and take preventative action.
Overall, digitalization will drive unprecedented levels of visibility, productivity and quality by increasing the connectivity across systems, enabling more collaborative manufacturing and data-driven decision making.
Catalyzing a culture shift
Turning data into intelligent decisions is the goal of digitalization, but to do that, companies must create the right culture. In my view, it is the culture within a company that is the main barrier to digitalization in the pharma industry, as opposed to the technical challenges. New technologies are slowly enabling new ways of thinking and operating, but people must be receptive and mindsets must evolve with the technology.
Oftentimes, people in pharma are very busy and focused on their immediate priorities: from getting products out of the door to good safety standards – digitalization might not be top of their agenda. However, I’ve also seen other companies who are very progressive, innovative and proactive in adopting new technologies and who are seeing real success stories from their use. I think it’s only a matter of time before digitalization becomes widespread within the pharma industry – the clear benefits will make it so. But the first step is perhaps to develop pilot studies or create user cases to demonstrate the value of digitalization to pharma businesses, before rolling out these new technologies and practices across sites. Of course, it would be remiss of me not to mention the regulatory challenges of implementing, say, an AI-based GMP solution for commercial manufacturing. Working to ensure new solutions are in line with regulatory requirements is an important challenge to overcome, but a lot of positive conversations are happening in this area.
We’ve only scratched the surface of what’s possible with digitalization. Technology continues to evolve, and the opportunities are almost endless. New technologies such as smart and wireless sensors that will transmit into your IoT platform to remotely monitor equipment, cloud computing, 3D printing, augmented and virtual reality will all be part of the digital revolution and I can’t wait to see where they lead the pharma industry.
Building the Brilliant Factory
Jun Huang believes we’re still scratching the surface of what’s possible with digitalization. As Chief Digital Officer at Cytiva, Ben Newton is also optimistic about the future. He believes that digital technology could be used to build “the brilliant factory.”
What developments have had the biggest impact in your field over the past five years?
I am excited by the increasing sophistication of cloud-based technologies, which allow us to compute large amounts of data in the cloud remotely. We are also seeing the emergence of technologies that extract data from patients or equipment through wearables and sensors. Then, we also now have the ability to aggregate that data in a structured way so that we can start to make predictions about disease and manufacturing methods (big data). Coupled with the digital revolution has been an increase in our knowledge of disease processes and how to use the immune system to treat cancer and this could have a real impact on how we address disease. It’s a fascinating time to be involved in the industry.
What is your vision for the future of manufacturing?
We need to bring all of the pieces that we are working on together under the roof of what we might call the “brilliant factory.” Right now, many in the biopharma industry are working on optimizing the cell culture and upstream process by developing smarter ways to define the right kind of process for the production of antibodies and cell therapies. We’re also defining the tools to separate and purify those antibodies cost-effectively with multivariate analytics tools. And to support manufacturing, we’re developing digital twins to optimize and control processes. At the moment, we’re doing these things somewhat in isolation but if we can bring them together in a single manufacturing setting, where every step is optimized, and the data is aggregated and analyzed by AI, you can create a system that can learn which factors are important for optimization and that is able to self-validate to automatically improve processes. This vision of a fully automated intelligent system is what we should be aiming for.