The ÄKTA pilot 600 chromatography system is designed to facilitate sanitization, with the high standards imposed by regulatory authorities for purity of the clinical products in mind. In this study, the predefined sanitization method developed for ÄKTA pilot 600 was assessed.
In a microbial challenge test, parts were exposed to a bacterial strain recommended by the United States Pharmacopoeia (USP 38) and to yeast strains commonly used in production environments.
Sanitization of the system was performed by running the predefined method using 1 M sodium hydroxide (NaOH) as sanitization agent. The results show excellent cleaning efficiency, with a log reduction factor of ≥ 6 for colony forming units (CFU) of the test microorganisms. Sanitization of the SNAP-connectors using 1 M NaOH was also successfully conducted.
Introduction
Bacteria and yeasts are found in many laboratory and production environments. Growing rapidly to large quantities under favorable conditions, these microorganisms can remain as contaminant of the bioproduct throughout manufacturing, with batch failure and related costs as a consequence.
Hence, it is important to follow hygienic routines throughout the whole production process. Sanitization is commonly used for chromatography systems to maintain microbial presence at levels that minimize the risk of contaminating the bioproduct. A few important terms that are related to system and production hygiene are explained in Table 1.
Table 1. Explanation of relevant and related terms
| Antimicrobial agents | Agents that minimize or destroy microorganisms in vitro. The term antimicrobial is general and all inclusive. Antimicrobial agents include sanitizers, sterilizers, and disinfectants. However, sanitizers, sterilizers, and disinfectants are not necessarily the same and sanitization, sterilization, and disinfection are not interchangeable terms. |
| CIP | Cleaning in place, where cleaning performed without disassembly of the equipment. |
| Disinfection | The destruction of potential pathogens (while cleaning is the removal of all kinds of contaminants, proteins, lipids, other particles, and microorganisms). |
| Sanitization | The use of chemical agents or steam to reduce a microbial population to acceptable, predetermined levels. |
| Sterile | Free from all living microorganisms. Antiseptics prevent microbial growth. Aseptic prevent bacterial entrance. |
| Sterilization | The act or process, physical or chemical, which destroys or eliminates all forms of life, especially microorganisms. |
ÄKTA pilot 600 is a bench-top chromatography system intended for process development, scale-up, and scale-down applications as well as in small- and intermediate-scale production. The system is designed to facilitate sanitization and is well-suited for use in both GMP and non-GMP environments (Fig 1). The system cabinet and the frontside with all valves and sensors are easily accessed to simplify external sanitization.

Fig 1. The ÄKTA pilot 600 chromatography system is designed to simplify the sanitization procedure, supporting biomanufacturers in meeting the high purity standards imposed by regulatory authorities for clinical products.
The stainless steel surfaces of the cabinet and the optional column stand can be wiped off and have minimal areas where dust and liquid can get trapped. In addition, the novel SNAP-connectors are easy to remove and omit the need for O-rings, which might otherwise present a sanitary weakness. For external cleaning, the air trap, rinsing solution module, and the optional filter module are easily removed, and the pegs left on the system are protruding to facilitate cleaning, thereby preventing collection of dust or liquid.
The wet side is designed to minimize system volume and dead-legs in the flow path. Materials are tested for extractables and leachables when in contact with solutions commonly used in the biomanufacturing industry. The predefined sanitization method for the flow path and the pump rinsing system requires minimal manual handling. The sanitization method is based on NaOH to allow for good process economy and bioburden control.
In this study, microbial challenge testing was performed to evaluate the efficiency of the predefined sanitizing method (1). The principle of microbial challenge testing is to introduce a high concentration of a predetermined microbial organism into the equipment, after which the challenged equipment is treated with a sanitization agent. After a specified time period, the number of surviving organisms are counted.
Here, challenge testing of ÄKTA pilot 600 was performed in duplicate using strains of both E. coli and yeast. After sanitization using NaOH, purified water was pumped through the system and the eluate was collected for evaluation of the system sanitization. In addition, the system was dismantled and sampled for remaining microorganisms at predetermined sites. Microbial challenge testing of the SNAP-connectors was performed separately.
Results from the sanitization study
Sanitization of the chromatography system
It is common practice to define sanitization as a 6 log reduction in CFUs achieved after a sanitization procedure. In this study, CFUs of the challenging organisms were counted at four phases during the sanitization study: in the inoculation solution, immediately after infection, prior to sanitization, and after sanitization. Results from determination of the concentrations of the challenging organisms in sample solutions are summarized in Table 1.
The inoculum or starting concentration of the challenging organisms was in the range of 9–14 × 106 CFU/mL. After leaving the system for 16 to 20 h at room temperature, the concentrations were measured again and was found to be between 8–17 × 106 CFU/mL. At this point, the sanitization method was conducted. Following sanitization, post-sanitization effluent analyses show no trace of viable challenging organisms.
Table 1. Challenging organism viable count
| E. coli, viable count (CFU/mL) | P. pastoris, viable count (CFU/mL) | |||
|---|---|---|---|---|
| Study 1 | Study 2 | Study 1 | Study 2 | |
| Inoculum, start concentration | 13.4 × 106 | 11.4 × 106 | 11.4 × 106 | 9.6 × 106 |
| Post-infection, effluent (outlet 6) | 12.8 × 106 | 10.1 × 106 | 12.2 × 106 | 7.8 × 106 |
| Pre-sanitization (after 16-20 h in RT), effluent (outlet 6) | 16.6 × 106 | 13.6 × 106 | 8.2 × 106 | 8.7 × 106 |
| Post-sanitization, effluent (outlet 1 to 6) | 0 | 0 | 1* | 0 |
*Growth of another organism than the challenging organism in 1 out of 6 outlets.
System sampling modules are shown in Figure 2. The results presented in Table 2 show that the sanitization method eliminated both E. coli and P. pastoris from the wet side of the system and from the pump rinsing system with a more than 6 log reduction. In these studies, the pump rinsing system was challenged with the same amount of challenging organisms as was the wet side, and a few CFUs of P. pastoris were detected in the pump rinsing system in the second study. To prevent growth in the rinsing system, the rinse solution should be exchange as recommended in the operating instructions.
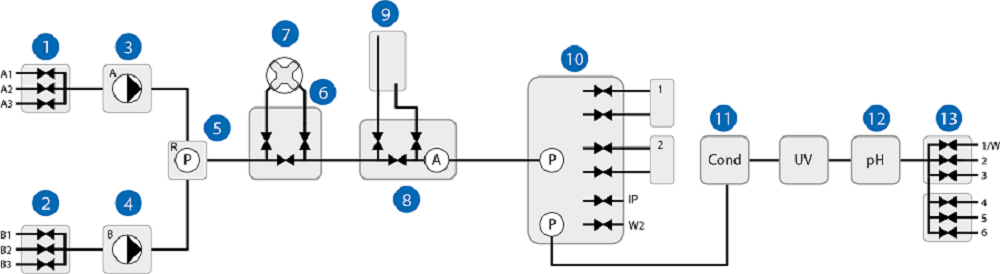
Fig 2. After sanitization using NaOH, purified water was pumped through the system and the eluate was collected for analysis. The system was also dismantled and sampled for remaining microorganisms at predetermined sites. All system modules are represented with one or more sampling points depending on the complexity of the module. *Due to its simple design, the component was tested as part of Test method 1.
Table 2. Number of CFUs remaining after sanitization with 1 M NaOH
| Sampling site | No. of sampling points | E. coli, viable count (CFU/mL) | P. pastoris, viable count (CFU/mL) | ||
| Study 1 | Study 2 | Study 1 | Study 2 | ||
| 1. Inlet A | 1 | 0 | 0 | 0 | 0 |
| 2. Inlet B | 1 | 0 | 0 | 0 | 0 |
| 3. Pump A | 8 | 0 | 0 | 0 | 0 |
| 4. Pump rinsing system | 8 | 0 | 1* | 0 | 4 + 1† |
| 5. Restrictor pressure sensor | 18 | 0 | 0 | 0 | 0 |
| 6. Mixer valve | 2 | 0 | 0 | 0 | 0 |
| 7. Mixer | 2 | 0 | 0 | 0 | 0 |
| 8. Air-trap valve | 10 | 0 | 0 | 0 | 0 |
| 9. Air-trap | 10 | 0 | 0 | 0 | 0 |
| 10. Column valve | 6 | 0 | 0 | 0 | 0 |
| 11. Conductivity monitor | 1 | 0 | 0 | 0 | 0 |
| 12. pH module | 4 | 0 | 0 | 0 | 0 |
| 13. Outlet | 1 | 0 | 0 | 0 | 0 |
*Growth of another organism than the challenging organism in 1 out of 8 sampling points.
†Growth of challenging organism in 2 out of 8 sampling points.
Sanitization of ÄKTA pilot 600 SNAP-connectors
Sampling sites are shown in Figure 3, and the results are presented in Table 3. No challenging organisms were found on any of the sampling points after sanitization in any of the studies performed. The results indicate that the SNAP-connectors were effectively sanitized.
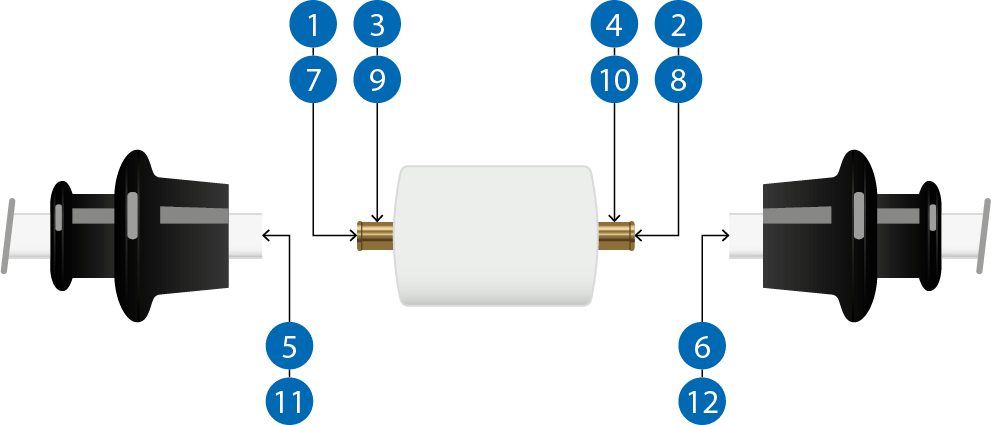
Fig 3. Sampling sites on the SNAP-connectors. For this study, two titanic pegs were inserted in a piece of peek-material.
Table 3. Number of CFUs remaining after sanitization with 1 M NaOH
| Viable count (CFU/mL) | ||
|---|---|---|
| Sampling site | E. coli | S. cerevisiae |
| Small connector | ||
| 1. Inlet, inside | 0 | 0 |
| 2. Outlet, inside | 0 | 0 |
| 3. Inlet, outside | 0 | 0 |
| 4. Outlet, outside | 0 | 0 |
| 5. Hose inlet, inside | 0 | 0 |
| 6. Hose outlet, inside | 0 | 0 |
| Large connector | ||
| 7. Inlet, inside | 0 | 0 |
| 8. Outlet, inside | 0 | 0 |
| 9. Inlet, outside | 0 | 0 |
| 10. Outlet, outside | 0 | 0 |
| 11. Hose inlet, inside | 0 | 0 |
| 12. Hose outlet, inside | 0 | 0 |
Conclusion from the study on ÄKTA pilot 600
The predefined chromatography system sanitization method was evaluated using two challenging organisms, a bacterial E. coli strain and the yeast P. pastoris. Analyses of remaining microorganisms in sample solutions as well as on sampled system parts show efficient sanitization of the system using the predefined protocol and 1 M NaOH as sanitization solution. Although a few CFUs of the challenging organism were detected in the pump rinsing system in one out of four studies, a more than 6 log reduction of the challenging organisms was achieved on the sampled system parts.
For the SNAP-connectors, a total of three sanitization studies were performed, using a bacterial E. coli strain and the yeast S. cerevisiae as challenging organisms. As shown for the wet side of the system, the results for the SNAP-connectors show no challenging organisms at any of the sampling points after sanitization.
To get more details on this study, download the ÄKTA pilot 600 sanitization application note.
Disclaimer
The information contained herein is not representative of any specific claims or any relevant environment, health, and safety laws and regulations, including use authorization, product registration or application licensing, or similar legal requirements.
Reference
- Current good manufacturing practices for finished pharmaceuticals, 21 CFR 211. [Online] www.fda.gov/ohrms/dockets/98fr/98110s1.pdf. Accessed 8 January 2018.