Bacterial endospores present a challenge in bioproduction due to their resistance to common sanitization procedures using high concentrations of NaOH. Several protein-based chromatography resins are also sensitive to such harsh sanitization conditions. As an alternative, the oxidizing agent peracetic acid (PAA) could be used in the control of such contaminants.
We describe an approach to safely sanitize our new generation BioProcess protein A affinity resin for antibody purification — MabSelect PrismA — with 30 mM PAA combined with NaOH cleaning in place regimen in a resin lifetime study.
The results of the study showed that:
- Sanitization of MabSelect PrismA with 30 mM PAA for 15 min at cycle 0, 40, and 80 could be performed for a total of 120 cycles with acceptable loss of yield and no significant effect on purity.
- Impurities such as aggregates and HCP levels were low and stable over the whole study.
- Results after 120 cycles support our recommendation of PAA treatment for 2 to 3 times per resin lifetime.
- PAA treatment in this study should only act as a guide. The sanitization frequency needs to be determined case by case.
Introduction
The wide range of sources of bacterial contamination in any bioprocess facility includes: staff employed at the facility, equipment, filter membranes, various process operations, gas contaminants and water, the chromatography resin, and numerous raw materials. Bioburden incidents resulting from contamination are to be avoided as they both stop productivity and lead inevitably to time lost in investigating the source of the contamination (1). The outcome: the facility will need to be sanitized and the entire production batch as well as the chromatography resin discarded. The demand for bioburden control from regulatory authorities is stringent as bacterial contamination can affect the safety and integrity of the bioproduct. If a sanitization protocol including the resin is considered it is important to make resin lifetime studies an essential part of your bioprocess development.
Some bacteria form highly resistant, dormant structures called endospores under conditions that are unfavorable for growth. The oxidizing agent PAA efficiently reduces bacterial spores, which are resistant to NaOH treatment. An example of this is in the treatment of MabSelect SuRe resin with 30 mM PAA for 15 min or 20 mM PAA for 30 min, which results in a more than 6 log reduction of Bacillus subtilis spores.
MabSelect PrismA is the latest development in our successful suite of protein A chromatography resins. The resin is an improvement of the base matrix and alkali-stable protein A ligand of MabSelect SuRe. Previous studies on MabSelect SuRe have shown that sanitization with 100 mM PAA for up to 24 h has no effect on the resin binding capacity and we have subsequently submitted a patent application for this sanitization method for affinity chromatography (2). This study summarizes our findings on tests with PAA on the performance of MabSelect PrismA in a resin lifetime study with a sanitization regimen with 30 mM PAA.
See the bottom of the page for detailed Materials and methods.
Results and discussion
Resin lifetime study
In an earlier study, we observed no significant effect on mAb yield (at 50% load of Qb10) or purification performance of MabSelect SuRe resin when treated with 20 mM PAA every fourth cycle for more than 100 cycles. Based on this study, we recommend PAA treatment two to three times per resin lifetime for MabSelect SuRe. We applied that recommendation to the present lifetime study where MabSelect PrismA was treated with 30 mM PAA at 0, 40, and 80 cycles up to 120 cycles at 80% load of Qb10. The control was the same process performed without including the PAA treatment step.
Our main findings from this approach were:
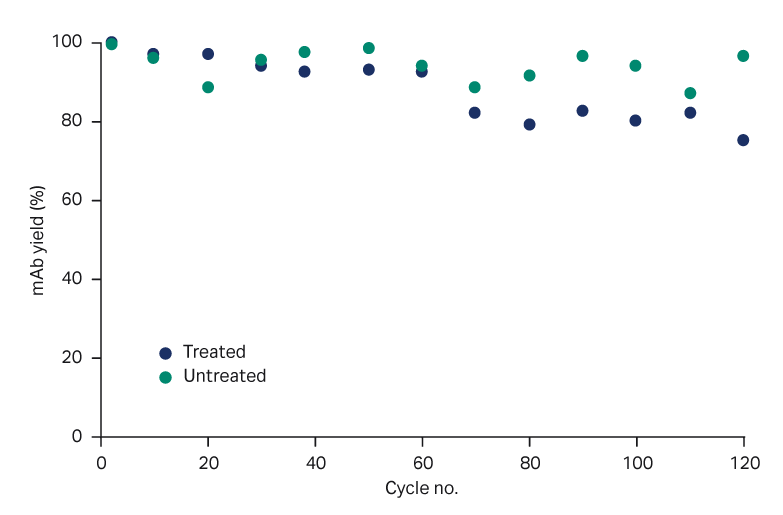
- Yield gradually decreased but was ≥ 75% for all cycles (Fig 1).
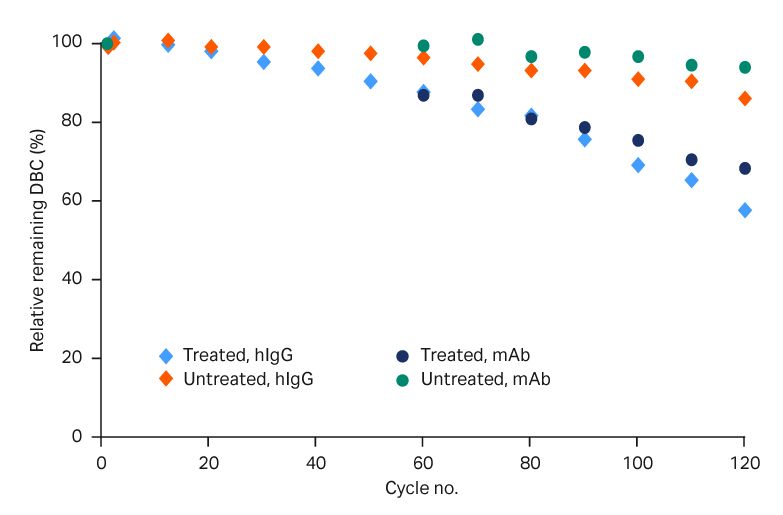
- DBC was reduced to 58% or 69% with polyclonal human IgG or mAb after 120 cycles (Fig 2).
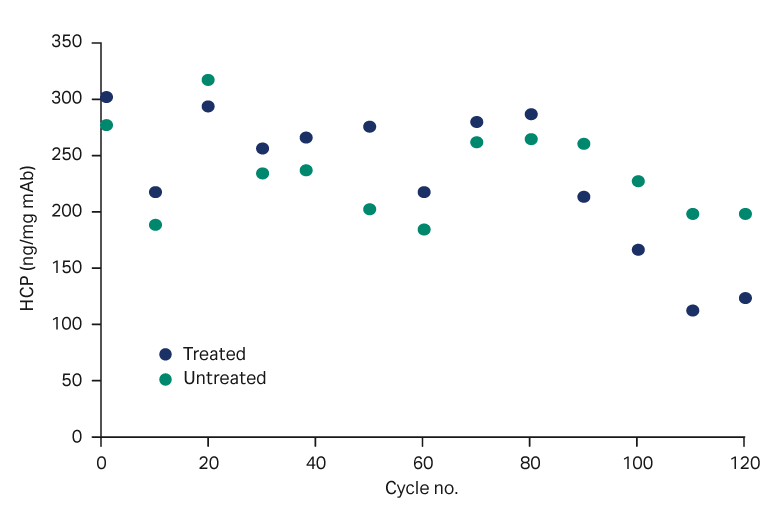
- HCP levels were similar for both the PAA-treated and untreated resin: between 100 and 300 for treated and between 200 and 300 ng HCP/mg mAb for untreated resin, respectively (Fig 3).
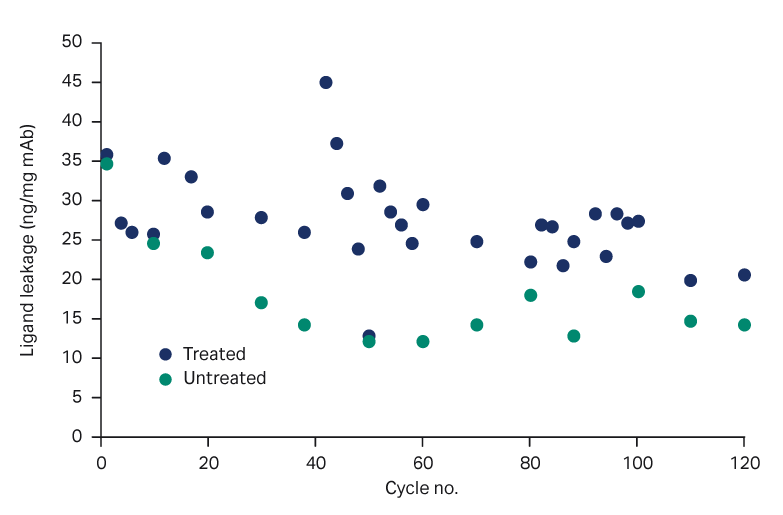
- Protein A ligand leakage was low at < 45 ng ligand/mg mAb for the PAA-treated resin and 35 ng ligand/mg mAb for the untreated resin, respectively (Fig 4). The PrismA ligand leakage was tested more frequently after PAA treatment at 40 and 80 cycles. Although a slightly higher leakage was observed, it was still < 45 ng/mg mAb.
Fig 1. Yield of mAb over process cycles, with and without PAA treatment.
Fig 2. DBC of hIgG and mAb over process cycles, with and without PAA treatment.
Fig 3. HCP content in process, with and without PAA treatment.
Fig 4. Ligand leakage, with and without PAA treatment.
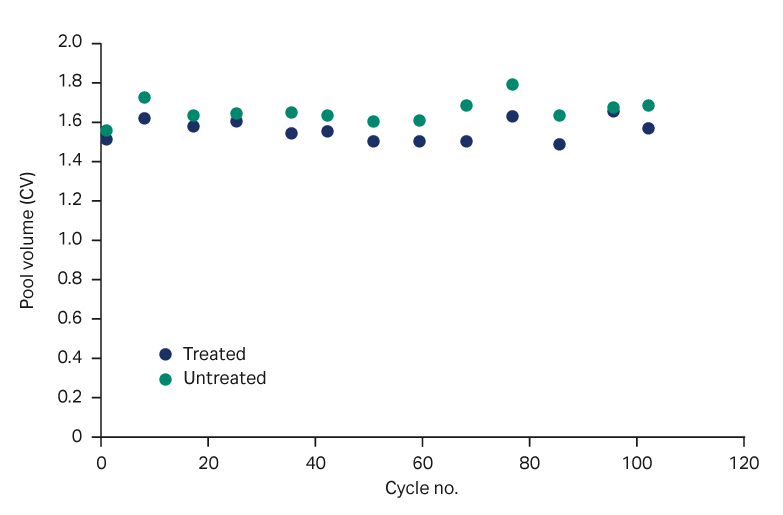
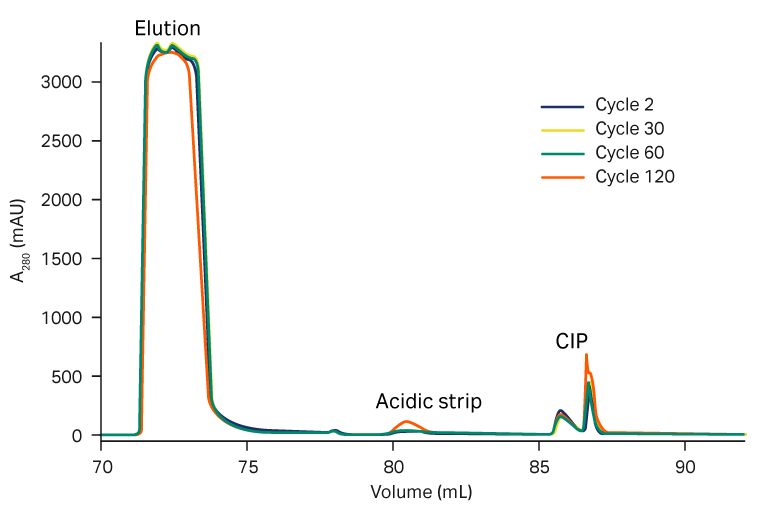
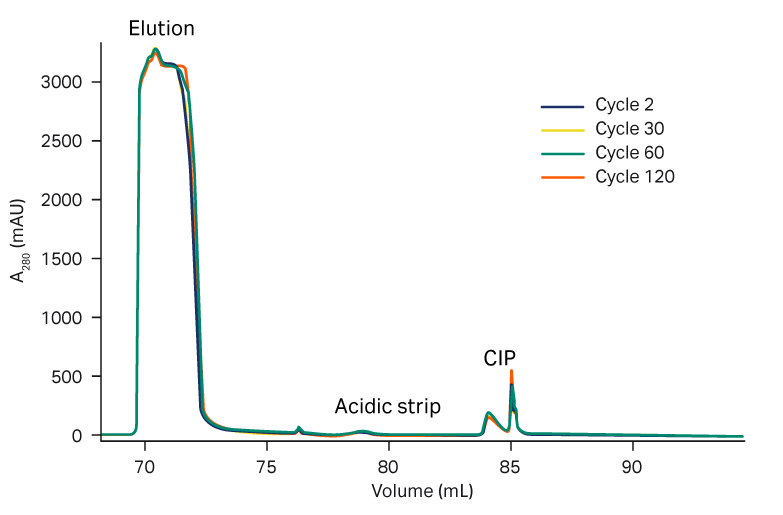
We observed a consistent elution pool volume, both with PAA treatment and without, up to 120 cycles (Fig 5). The strip and CIP peaks were consistent over the cycles (Fig 6).
Fig 5. Elution pool volumes over process cycling at load 80% of Qb10, with and without PAA treatment.
A)
B)
Fig 6. Overlay of elution, acidic strip, and CIP peaks over process cycles in (A) with and (B) without PAA treatment.
Compatibility of PAA with equipment materials
PAA is a strong oxidizing agent and care should be taken during handling of this chemical. You should also consider the compatibility of the equipment you use in processes including PAA treatment.
Information from the literature and from our internal studies on PAA compatibility of materials used in wetted parts of our BioProcess hardware and single-use components is given in Table 1.
Table 1. Resistance to PAA of materials used in wetted parts of manually packed AxiChrom and BPG columns as well as in prepacked ReadyToProcess columns and single use ÄKTA ready flow kits
| Material | Comments |
| Acrylate | Resistant to PAA |
| Fluoroelastomer (FKM) | |
| Fluoroethenepropylene (FEP) | |
| Fluorocarbon rubber (FPM) | |
| Platinum-cured silicone | |
| Perfluoro elastomer (FFKM) | |
| Polyethylene (PE) | |
| Polyoxymethylene (POM) | |
| Polypropylene (PP) | |
| Polystyrene (PS) | |
| Polytetrafluoroethylene (PTFE) | |
| Polyvinylidene difluoride (PVDF) | |
| Silicone (SU) | |
| Stainless steel (SS) | |
| Tygon™ 2275 | |
| Ultra-high molecular weight polyethylene (UHMWPE) | |
| Borosilicate glass (BG) | No information on compatibility with PAA, but compatible with hydrogen peroxide |
| Polyether ether ketone (PEEK) | |
| Polymethylpentene (TPX) | |
| Ethylene propylene diene monomer (EPDM) | Some effect observed but compatible with 100 mM PAA up to 24 h |
| Polyamide (PA) | |
| Thermoplastic elastomer (TPE) | |
| Polyurethane (PU) | Some effects observed especially at high PAA concentration |
Based on Table 1, materials used in wetted parts of AxiChrom and BPG columns as well as ReadyToProcess columns and ÄKTA ready flow kits are considered compatible with PAA under conditions used in this work (20 mM for 30 min or 30 mM for 15 min).
Polyurethane, used in for instance the BPG column stand, is not compatible with PAA and you should avoid exposure of the stand to PAA during use.
Conclusions
The results from our long-term lifetime study for MabSelect PrismA support our recommendation of PAA treatment for two to three times per resin lifetime. Our additional conclusions from the study are:
- Sanitization of MabSelect PrismA with 30 mM PAA for 15 min at cycle 0, 40, and 80 could be performed for a total of 120 cycles with acceptable loss of yield and no significant effect on purity. PAA could therefore offer you a solution for bioprocess decontamination of spore-forming bacteria.
- The final decision on sanitization protocol will depend on the safety margin during mAb load and the number of cycles that the protein A resin will be used for before discarded, that is, the useful lifetime of the resin.
As a general guideline, sanitization with PAA could be performed before column use and between campaigns. In case of a bioburden incident for remediation of the resin, two to three treatments during the resin lifetime are recommended. Before applying PAA treatment, be sure to take the compatibility of process equipment, such as column hardware and single-use components into account.
References
- Suvarna, K., et al. Case studies of microbial contamination in biologic product manufacturing. American Pharmaceutical Review 14, 50–56 (2011).
- Monie, E. M. et al. Sanitization method for affinity chromatography matrices. World Intellectual Property Organization, Publication no. WO2016139128 A1, 9 Sep. (2016).
Materials and methods
1. Sample preparation
Before the MabSelect PrismA cycling, we filtered the clarified harvest containing internally produced mAb (IgG1) at a concentration of 2.1 g/L using an ULTA Pure HC 0.2 µm filter.
2. Experimental setup
MabSelect PrismA resin was packed in Tricorn 5/100 columns to a bed height of 10 cm, which corresponds to a column (CV) of 2 mL.
The columns were connected to an ÄKTA pure system.
We executed the MabSelect PrismA lifetime study over 120 cycles (Table 2). We loaded at 56 mg of mAb/mL resin, which corresponds to 80% of the dynamic binding capacity (DBC) at 10% breakthrough (Qb10) with a residence time of 6 min. CIP was performed with 0.5 M NaOH. Packed columns were exposed to 30 mM PAA for 15 min at cycle 0, 40, and 80 (Table 3). The process for the untreated resin was the same but without PAA treatment over 120 cycles.
Table 2. Process conditions used in the MabSelect PrismA purification step
| Phase | Buffer | Volume (CV) | Residence time (min) |
| Equilibration | 20 mM sodium phosphate, 150 mM NaCl, pH 7.4 | 3 | 4 |
| Sample load | 80% of Qb10 value for MabSelect PrismA | – | 6 |
| Wash 1 | 20 mM sodium phosphate, 500 mM NaCl, pH 7.0 | 5 | 6 |
| Wash 2 | 50 mM sodium acetate, pH 6.0 | 1 | 4 | Elution | 50 mM sodium acetate, pH 3.5 | 3 | 12 | Strip | 100 mM acetic acid, pH 2.9 | 3 | 4 | Cleaning in place (CIP) | 500 mM NaOH | 3 | 5 | Re-equilibration | 20 mM sodium phosphate, 150 mM NaCl, pH 7.4 | 3 | 4 |
Table 3. PAA treatment was performed three times during the lifetime study: before the study started, after cycle 40 and cycle 80
| Phase | Buffer | Volume (CV) | Residence time (min) |
| Prewash | Milli-Q™ water | 7.5 | 30 | PAA treatment | 30mM PAA | 3.75 | 15 | Wash 1 | Milli-Q water | 7.5 | 30 | Wash 2 | 20 mM sodium phosphate, 150 mM NaCl, pH 7.4 | 7.5 | 30 |
3. Analyses
We evaluated DBC by frontal analysis using a polyclonal human IgG (hIgG, Gammanorm™) before the lifetime study started. Our evaluation continued every 10 cycles.
In the frontal analysis, we diluted IgG in equilibration buffer to 2.0 mg/mL. To obtain a maximum UV absorbance value at 280 nm, we by-passed the column with the IgG solution.
After equilibration of the MabSelect PrismA column, we loaded hIgG until breakthrough using a flow rate corresponding to a residence time of 6 min. Unbound hIgG was washed off with equilibration buffer. Bound hIgG was eluted with acetic acid, pH 2.9. Qb10 was determined from the breakthrough curve, using a standardized spreadsheet.
To determine mAb yield during the lifetime study, selected eluates were analyzed for mAb content using either absorbance measurement or Biacore T200 surface plasmon resonance system.
We analyzed host cell protein (HCP) concentration in the product pool using commercially available CHO HCP E3G kit (Gyros Protein Technologies). Leakage of ligand was determined using a commercially available ELISA kit (Repligen Corp.) using MabSelect PrismA ligand as standard.
Disclaimer
The results and conclusions presented in this application note are valid for this specific study only. Other study conditions could have significant impact on the outcome. The method for sanitization using PAA should be decided case by case and lifetime of the packed column should be evaluated for each process. The overall finding is that MabSelect PrismA can be treated with 30 mM PAA for 15 min. Sanitization with PAA should not replace CIP with NaOH. The information contained herein is not representative of any specific claims or any relevant environment, health, and safety laws and regulations, including use authorization, product registration or application licensing, or similar legal requirements.
TR29627766