By: Takuo Kawase, Senior Manager and Scientist of the Downstream Process Development group at Chugai, a member of the Roche group
The last few years have seen a shift in antibody therapeutic pipelines towards more targeted patient populations and diverse antibody modalities. This has resulted in increased demand for smaller batch sizes, and flexible modular bioprocessing. Given this change, the industry has focused on creating flexible manufacturing solutions that can minimize changeover time between campaigns and maximize facility utilization.
My team evaluated Fibro rapid cycling chromatography for its performance and scalability for our downstream chromatography processes.
Introduction
As the biopharma industry moves towards more diversified pipelines of biological modalities and targeted patient populations, the demand for cost-efficient manufacturing becomes more challenging. There are increasing demands for flexible single-use solutions in manufacturing facilities. In commercial manufacturing, single-use solutions have been implemented to a large degree in upstream operations, but in downstream chromatography the general implementation is still lagging.
One of the key obstacles so far for implementation of true single-use chromatography is cost. The diffusion restricted flow rates in resins prevents efficient utilization of the full lifetime within a single batch run. To achieve cost efficiency, resins need to be reused over a number of batches. Existing alternatives like monoliths and membranes, on the other hand, offer desired flow rates but have so far shown low binding capacities. Fiber-based chromatography, i.e., Fibro PrismA, with immobilized Protein A ligand, offers the advantage of having the desired flow rates with high binding capacities. This will offer opportunities for rapid cycling chromatography that enables faster throughput in process development and single-use chromatography with full utilization of the unit lifetime within a single batch.
Evaluating the performance of Fibro PrismA
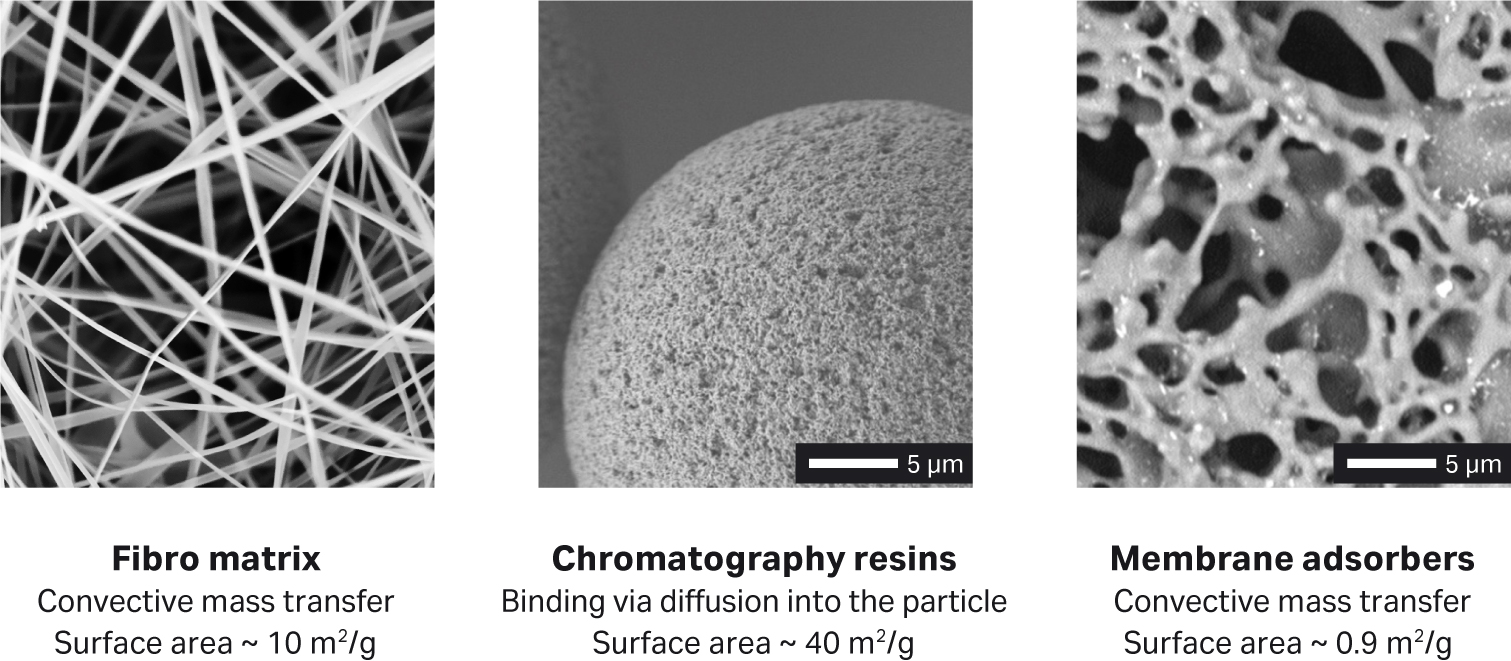
Fibro chromatography uses a well-defined matrix of cellulose fibers that has an open structure. The proprietary structure allows the technology to overcome the diffusional and flow limitations of packed bed chromatography purification, as well as the capacity issues of membrane adsorbers (Fig 1). As Fibro chromatography is expected to dramatically increase the throughput of Protein A chromatography, we first evaluated its performance with different antibodies.
Fig 1. Surface area and mass transfer mechanisms for different chromatography base matrices.
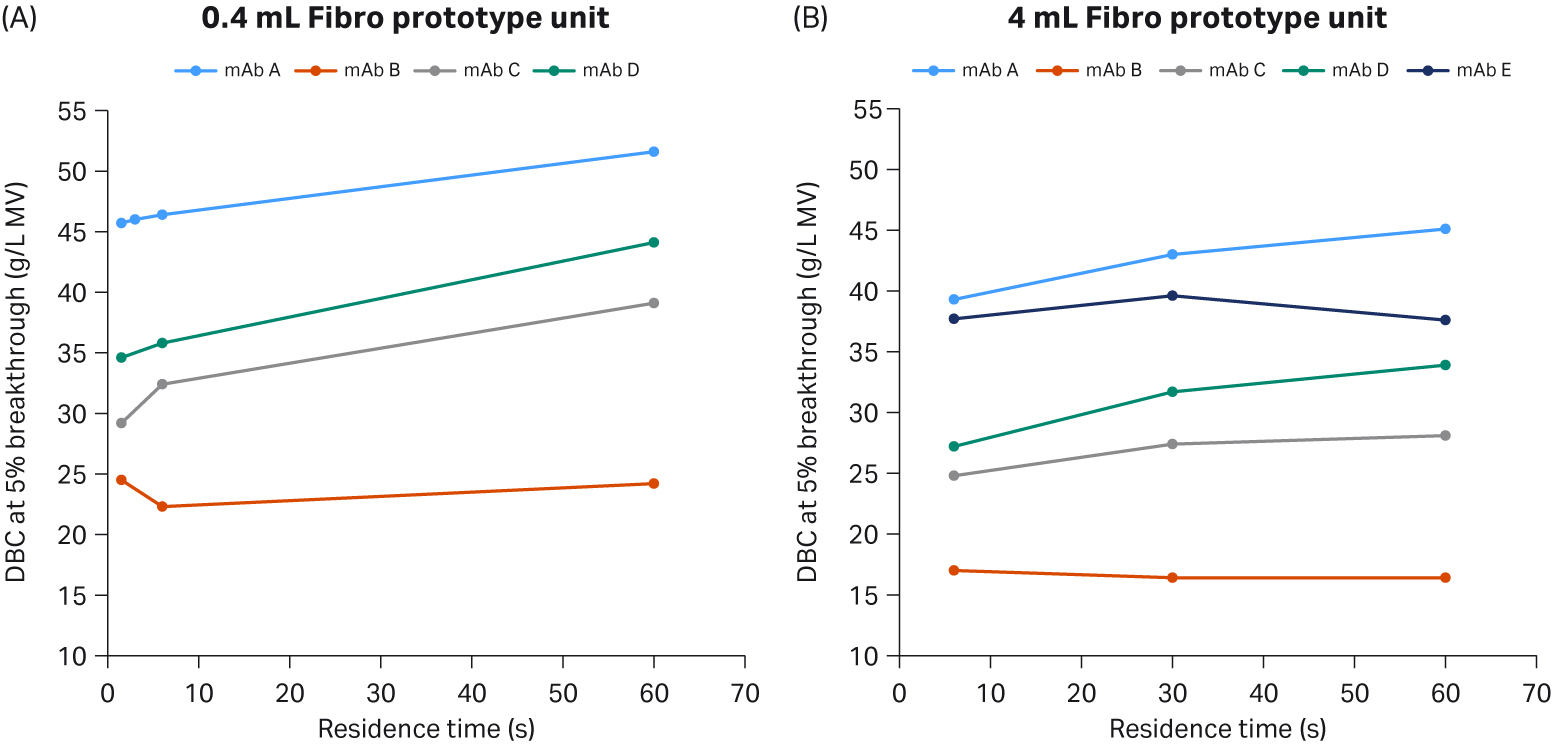
We evaluated the dynamic binding capacities (DBC) of a 0.4 mL prototype unit and a 4 mL Fibro prototype unit with five different antibodies and residence times up to 60 seconds (Fig 2). Results showed high DBC for both units with up to 45 mg/mL within a few seconds residence time.
Fig 2. DBCs and residence times up to 60 seconds of 0.4 mL and 4 mL Fibro units for five different antibodies.
Comparison of chromatography resins and Fibro throughput
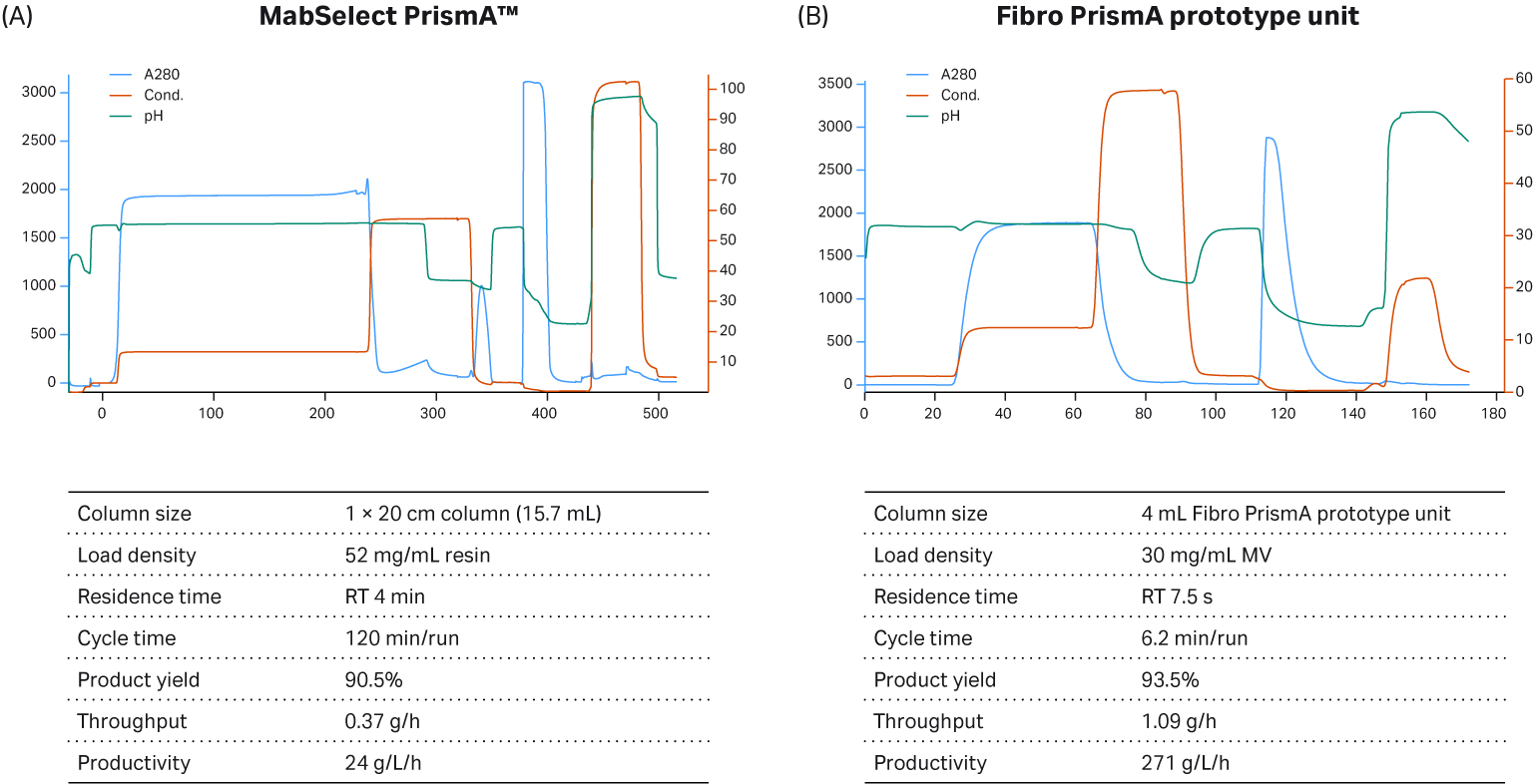
Next, we evaluated the purification throughput of a 15.7 mL MabSelect PrismA™ column and the 4 mL Fibro PrismA prototype unit (Fig 3). The purification cycle time for the MabSelect™ resin column was two hours, while the cycle time of the Fibro PrismA unit was 6 minutes due to its short residence time. This means that the Fibro unit can purify more than 2.9 times the amount of antibody within the same period of time as the MabSelect PrismA™ column. Or, that the unit size can be reduced by more than 1/10 for the Fibro unit in comparison to a resin column.
Fig 3. Comparison of throughput of MabSelect PrismA™ column to 4 mL Fibro PrismA prototype unit.
Fibro lifetime study
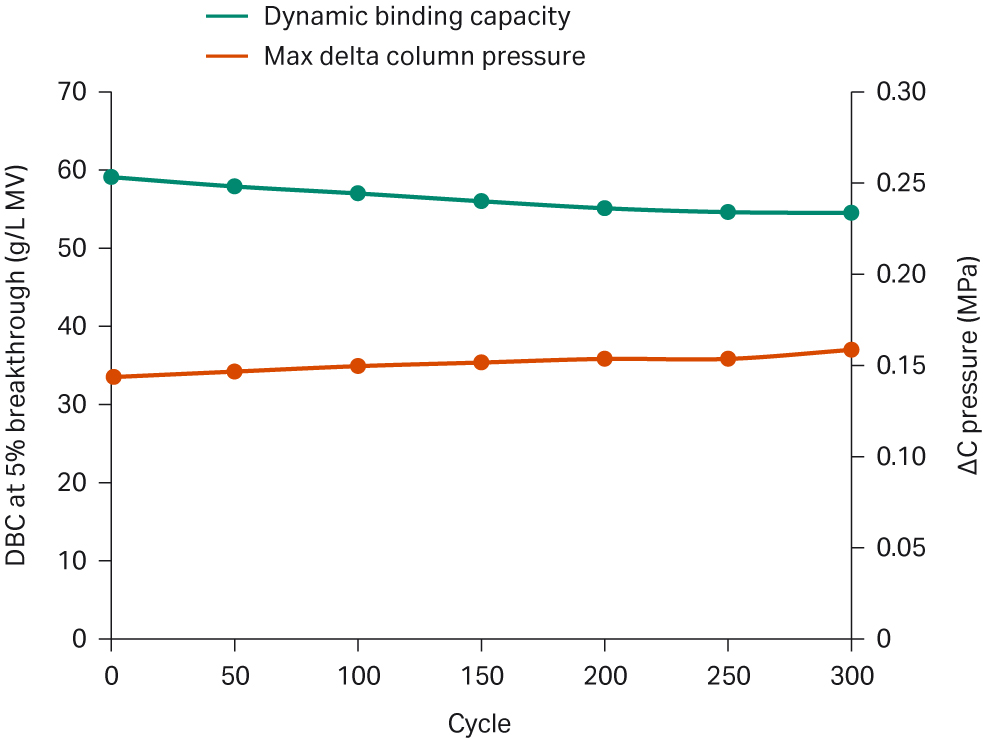
Then we performed a lifetime study of the 0.4 mL small-scale Fibro unit using an ÄKTA™ avant 25 system (Fig 4). Initially, the Fibro PrismA had a DBC of 59 g/L at 5% breakthrough for this antibody. The DBC of the Fibro PrismA unit was tracked every 50 cycles up to 300 cycles. Each cycle consisted of loading at 30 g/L MV with a residence time of 7.5 seconds, and a 60 second reverse flow 1 M NaOH clean in place (CIP) step. After 300 cycles, the Fibro PrismA unit retained more than 90% of its initial binding capacity without any notable pressure increase.
Fig 4. DBC of 0.4 mL Fibro PrismA unit over 300 cycles.
Scaling up Fibro PrismA for downstream processing
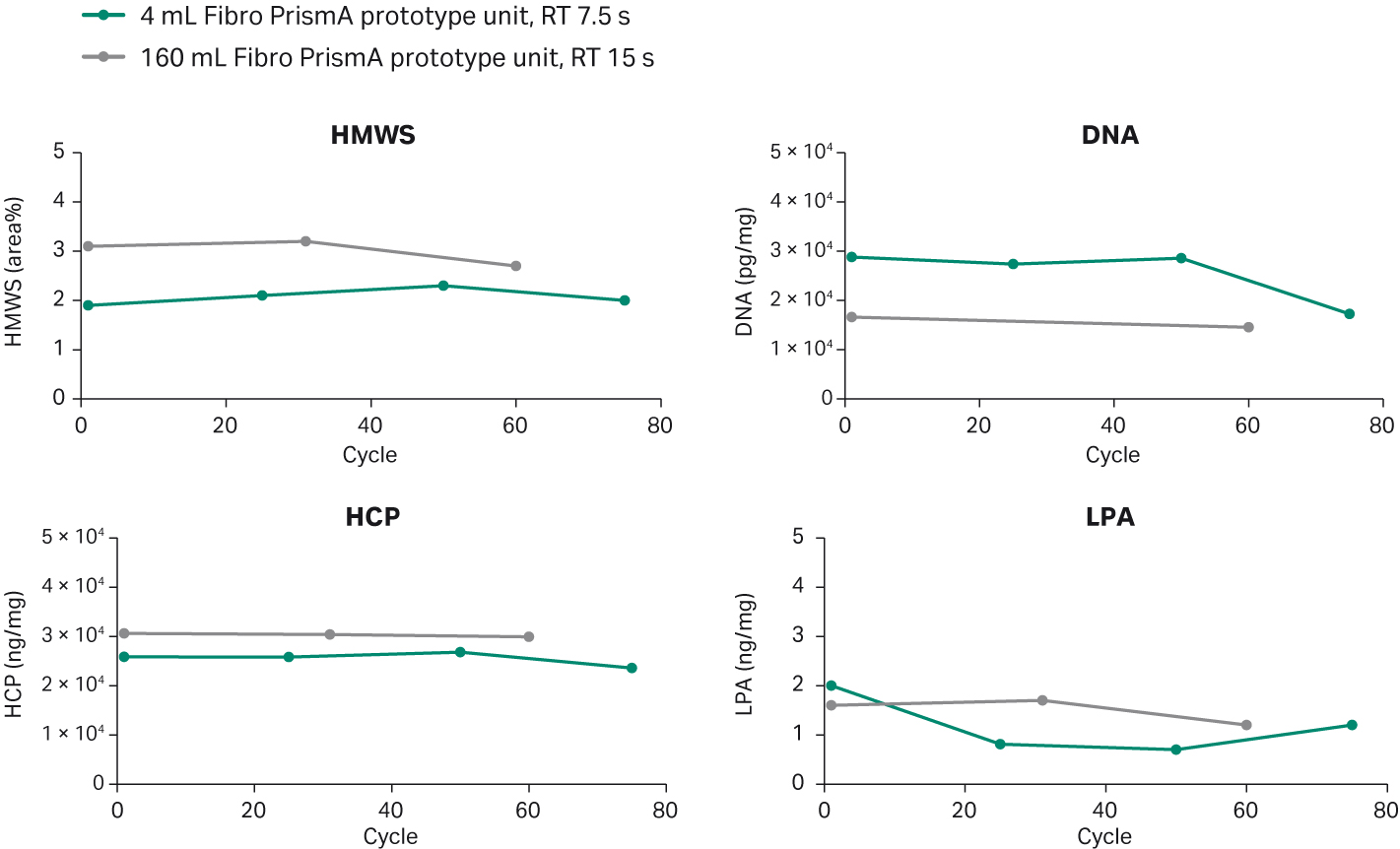
Finally, we evaluated the ability to scale-up Fibro PrismA using the 4 mL prototype unit on an ÄKTA™ avant 150 system and a 160 mL prototype on an ÄKTAprocess™ system. We evaluated the 4 mL unit over 75 cycles, and the 160 mL unit over 60 cycles in parallel, scaling the method with the same residence time (process performance study) and number of MVs at both scales. To evaluate the performance of the two units, we measured the high molecular weight (HMW) aggregates, host-cell proteins (HCP), residual DNA, and Protein A leakage (Fig 5). There was no significant difference between the two units, and the values were also consistent across the number of cycles.
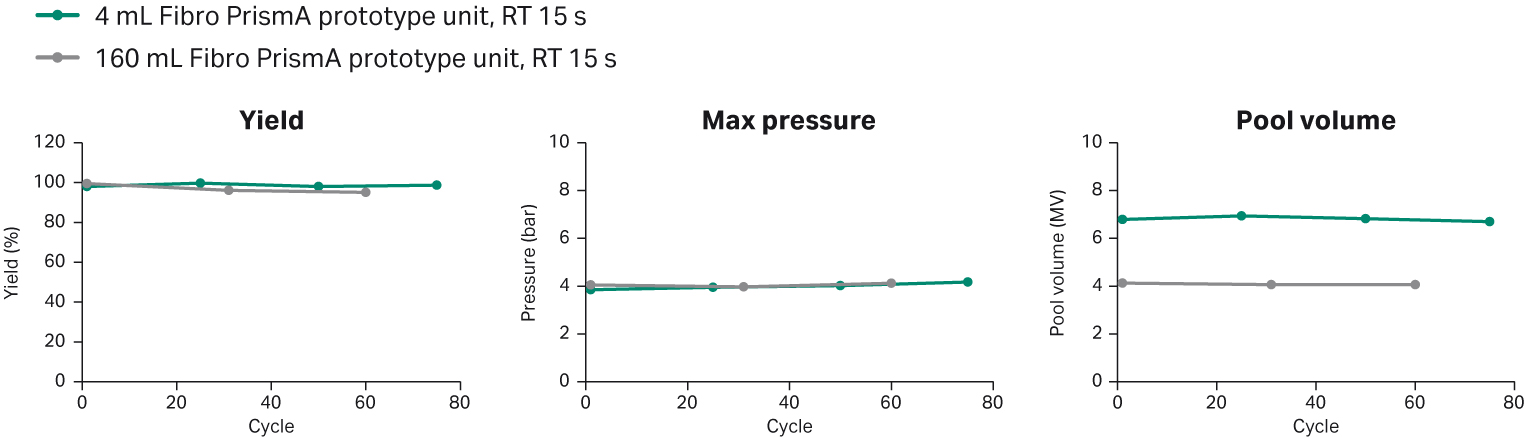
We also evaluated the process performance for both units across the study (Fig 6). The product yield and the back pressure were similar for the 4 mL unit and the 160 mL prototype. However, the pool volume differed between the two units. The reason for the difference in pool volume was caused by the difference in flow distribution structure between the 4 mL and the 160 mL units and the relative influence of system dead volumes. This showed that further optimization for pool volume and buffer usage was needed for implementation at large-scale, which we performed next.
Fig 5. Purification performance of 4 mL Fibro Prisma unit versus 160 mL Fibro Prisma prototype unit.
Fig 6. Process performance of 4 mL Fibro Prisma unit and 160 mL Fibro Prisma prototype unit over study.
Optimizing process conditions for Fibro PrismA
To optimize process conditions, it is important to understand the difference between resins and Fibro PrismA. We found that in some cases the same purification protocols as resins can be used with Fibro PrismA with great results. However, in other cases due to differences in the system and unit dead volumes between resin packed columns versus Fibro PrismA you will need to adapt process conditions.
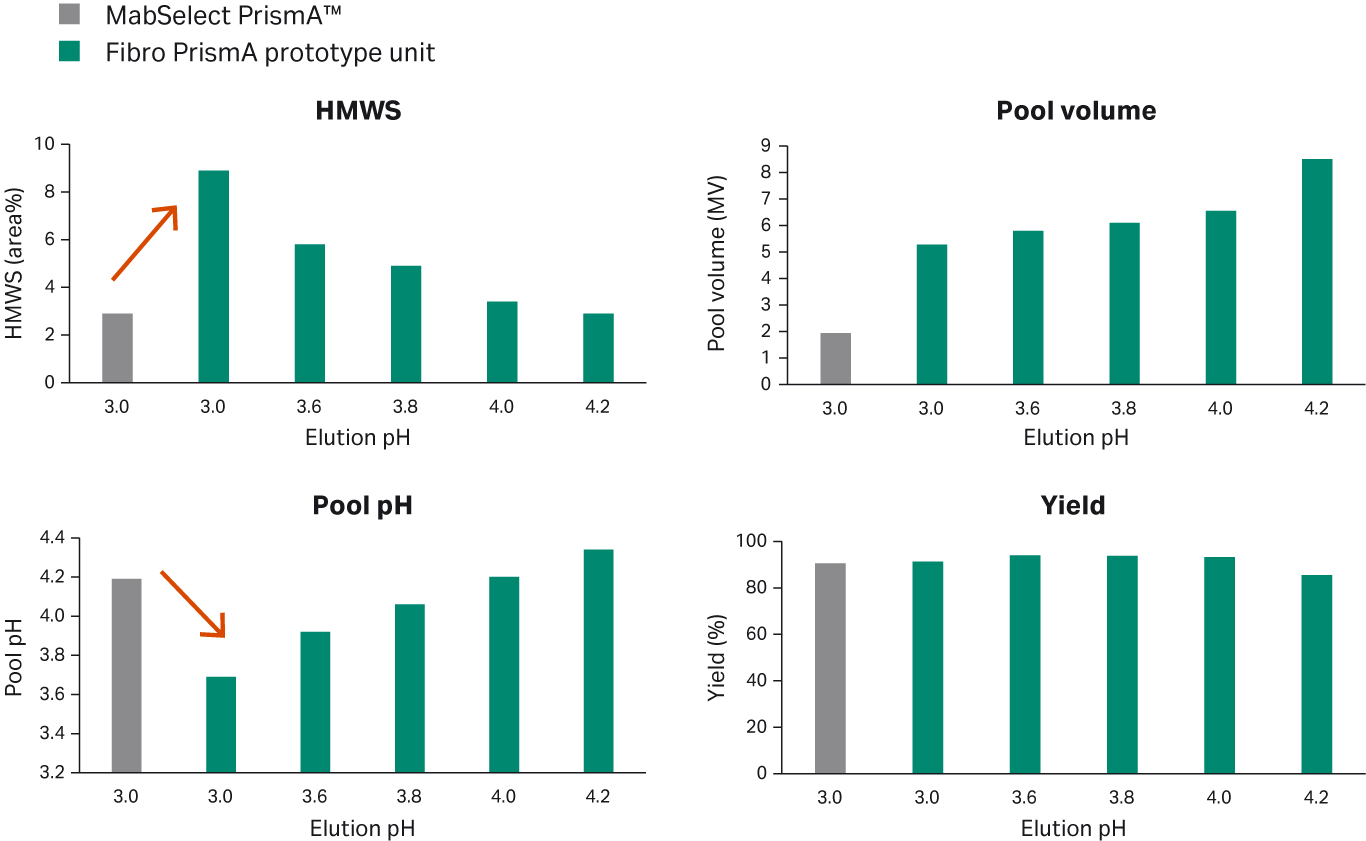
For example, for resins we typically use 50 mM sodium acetate at pH 3.0 as our protein elution condition. When we purified a sample with MabSelect PrismA™, the aggregate level was 2.9% in the elution pool. When the same purification protocol was used with Fibro PrismA, the aggregate level increased to 8.9%. This increase was caused by the lower elution pool pH, with the larger pool volume (Fig 7). In our case we needed to balance aggregate level with pool volume.
Fig 7. Case study showing the need for process optimization for Fibro PrismA units when using the same purification protocol as resins.
For this reason, when implementing Fibro PrismA we found you will need to consider diffusion, system hold up volume, wash buffer carryover, and resolution when optimizing your process. The advantage when optimizing conditions for Fibro PrismA is that its fast purification time make it easy to optimize process conditions quickly, accelerating the process development timeline.
An important consideration in large-scale manufacturing is the system hold up volume. The relatively smaller Fibro unit volume needed to purify a certain amount of antibody compared to a column, increases the impact of system hold up volume. A larger system volume will result in two negative effects. First, greater buffer usage is needed for each chromatographic step to clear the larger dead space. Second, there will be increased mixing of buffer phases, which will result in broader phase transitions, and therefore, broader elution peaks. Furthermore, the elution pool volume is also increased due to the relatively larger dead volume inside the Fibro unit.
This will result in larger elution pool volumes, which could impact the product critical quality attributes (CQAs) and affect facility fit. For these reasons, pairing the right system with each Fibro unit size is critical to ensuring optimum performance in terms of buffer usage and elution pool volume. For the same reasons, it is important to minimize the volume of tubing connecting the Fibro unit to the system. Smaller volume tubing will lead to sharper buffer transitions, due to the reduced mixing between buffer interfaces. By optimizing these parameters, the elution pool volume for large-scale manufacturing using Fibro chromatography can be reduced. To test this, we simulated a 2 000 L scale manufacturing operation based on the pilot scale evaluation data.
Manufacturing simulation 2.4 L Fibro PrismA and ÄKTAprocess™ unit
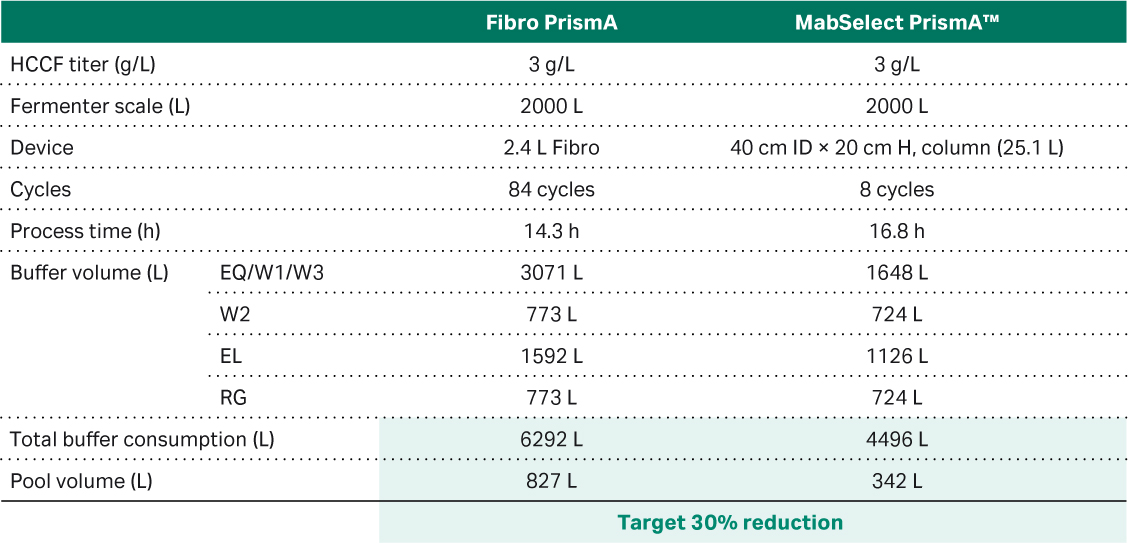
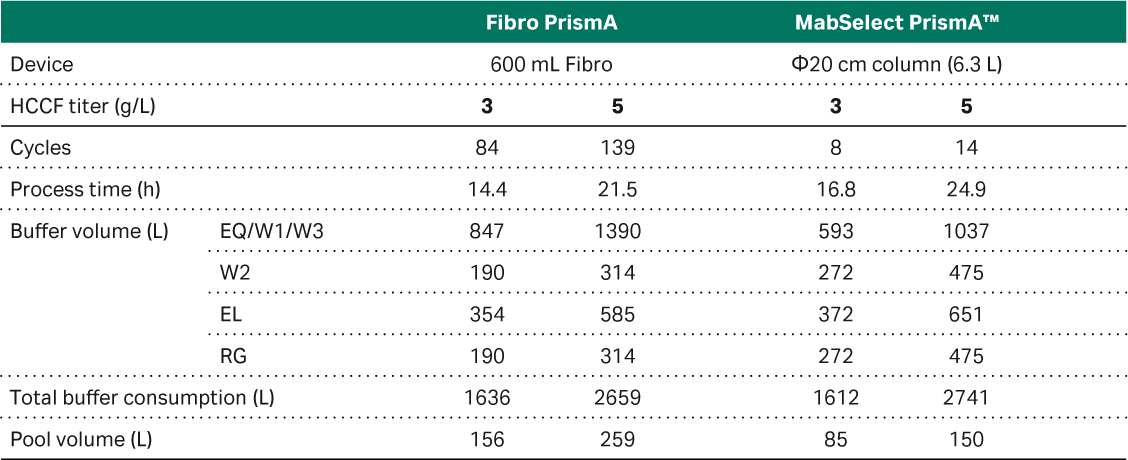
Finally, we wanted to simulate buffer consumption and elution pool volume in a large-scale manufacturing scenario with a 2.4 L Fibro PrismA prototype process unit and ÄKTAprocess™ system (Table 1). The ÄKTAprocess™ system was used as this is the existing chromatography system used in our manufacturing facility. We compared the result of the simulation with a packed resin in a 25 L column, which showed an almost 50% increase in total buffer consumption for the Fibro unit and more than doubling of the elution volume (see Pool volume in Table 1). This was a potential concern for implementing Fibro in our large-scale manufacturing, due to facility fit within our manufacturing suite. To address this concern, we performed an additional study using the 160 mL Fibro PrismA prototype connected with a low flow kit to an ÄKTA™ ready single use system. This set up allows for a true single-use solution for large-scale manufacturing, as well as a chromatography system with a smaller and more appropriate dead volume to the 160 mL Fibro unit.
Table 1. Results of a simulation of a large-scale process using a 2.4 L large scale Fibro prototype unit and 25 L MabSelect™ PrismA column connected to a ÄKTAprocess™ 10 mm I.D. polypropylene system.
Note: Column procedure uses system wash while Fibro procedure skips that step
For this study we optimized the following three parameters:
- Elution pH
- Elution priming step, system line is flushed with elution buffer at the beginning of elution phase
- Residence time
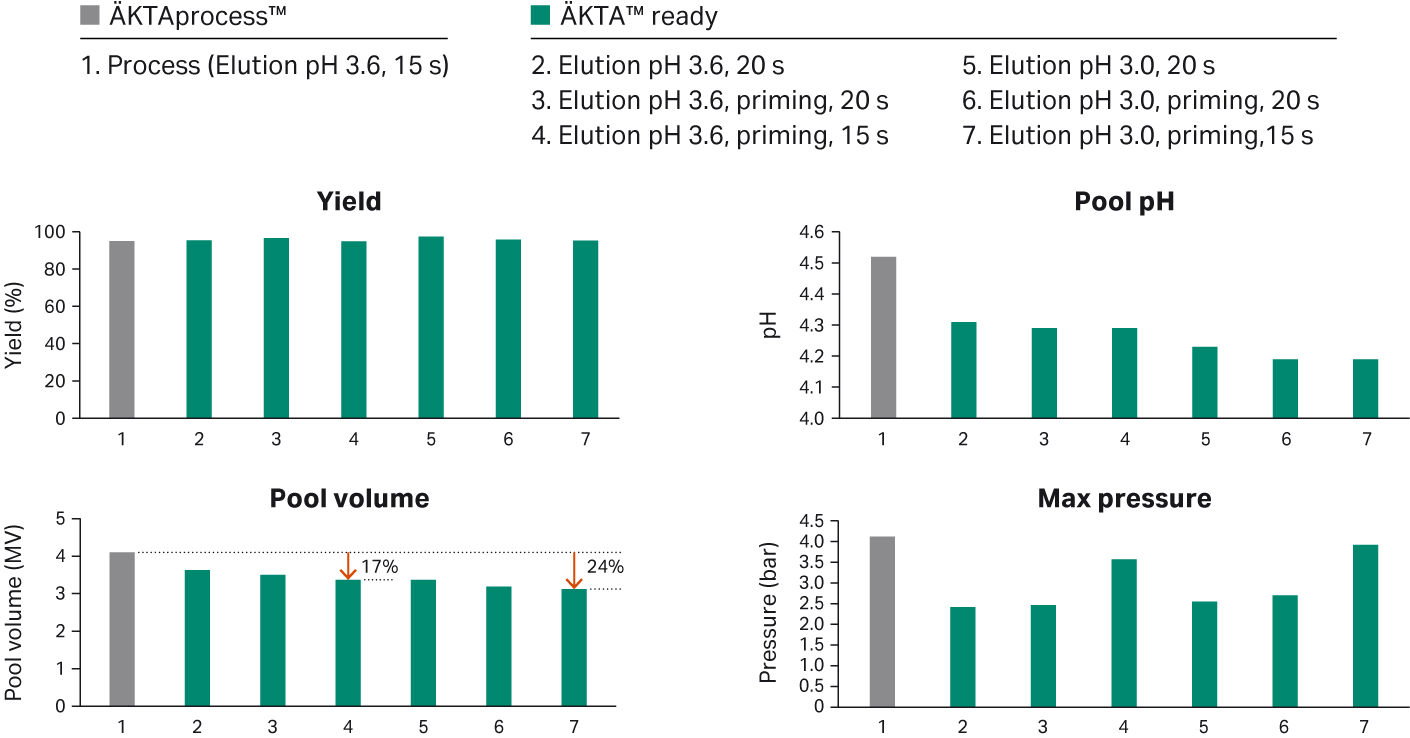
Results of optimizing of the process on a 160 mLFibro PrismA prototype unit and ÄKTA™ ready system with a low volume flow kit showed that the elution pool volume could be reduced with a consistent yield (Fig 9). The best results from this study were obtained with an elution buffer at a pH of 3.0, system priming, and a residence time of 15 seconds. With these conditions the elution pool volume could be reduced by 24% while keeping the elution pool pH at a level preventing aggregate formation.
Fig 8. Optimization study for reducing elution pool volume while keeping a consistent yield.
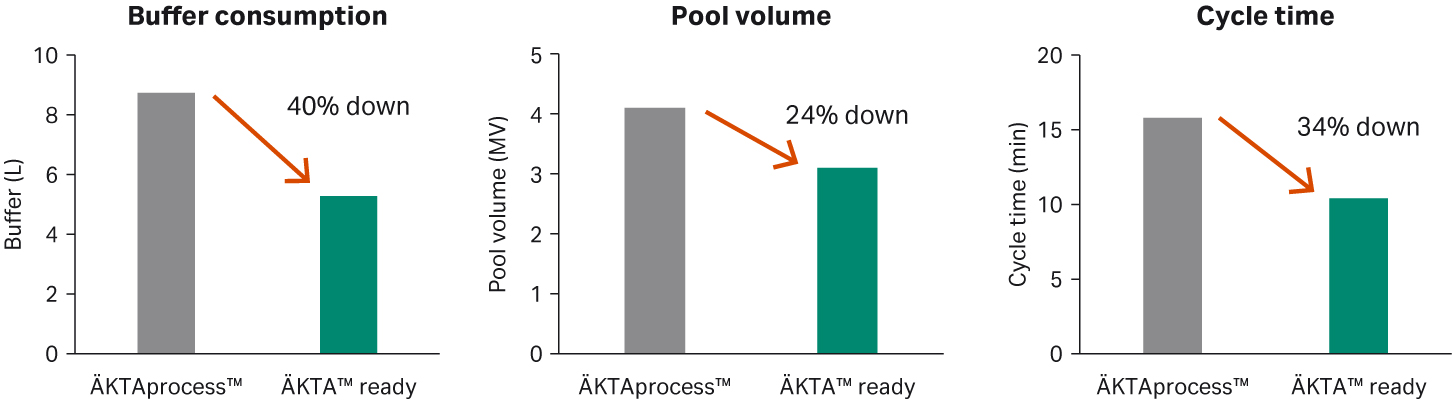
Compared to running the 160 mL Fibro PrismA unit on a ÄKTAprocess™ 10 mm system, both pool volume and buffer consumption could be significantly reduced, 24% and 40% respectively, illustrating the importance of optimizing the run conditions and matching the correct size Fibro unit with chromatography system.
Fig 9. Optimizing buffer consumption, pool volume and cycle time using a chromatography system with run conditions and hold up volumes more fit for the specific Fibro unit size.
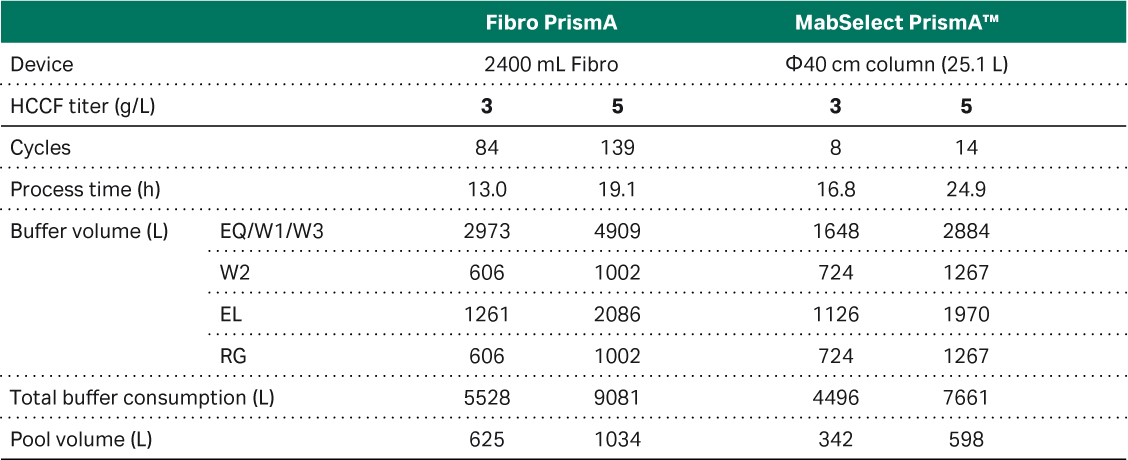
Based on the ÄKTA™ ready system evaluation a new simulation of performance in our manufacturing suite was performed. Here we can see that both pool volume and buffer consumption could be reduced for Fibro units (Tables 2 and 3). In the 500 L bioreactor simulation similar results were obtained with Fibro versus the column when it comes to buffer consumption, while pool volume was still 70-85% higher for Fibro units. In the 2 000 L simulation, there were still smaller pool volumes and buffer consumption with a column, especially for the high titer case (5 g/L). However, the Fibro unit values were within acceptable volumes for our processes. Note, the Fibro PrismA units up to 2.4 L unit size can be run on the ÄKTA™ ready system for process scale, optimizing elution volume and buffer volume further. This was not performed here as the simulation was performed for the ÄKTA™ process unit available in the production facility.
Table 2. Results of a simulation of a 500L bioreactor harvest feed using a 600 mL Fibro prototype unit and 25 L MabSelect™ PrismA column connected to a ÄKTA process 10 mm I.D. polypropylene system.
Note: Column procedure uses system wash while Fibro procedure skips that step
Table 3. Results of a simulation of a 2 000 L bioreactor harvest feed using a 2.4 L Fibro prototype unit and 25 L MabSelect™ PrismA column connected to a ÄKTAprocess™ 10 mm I.D. polypropylene system.
Note: Column procedure uses system wash while Fibro procedure skips that step
Conclusion
In summary, we found from the cycle lifetime studies that the 0.4 mL and 4 mL Fibro PrismA prototype units had a stable and consistent purification performance up to 300 cycles. Next, we were able to successfully scale-up our purification process from 4 mL to 160 mL Fibro PrismA prototype unit without any deviations. From the scale-up evaluation it became apparent that it is important to consider the differences between resins and Fibro to optimize your process. Particularly, optimizing the running conditions and pairing the right chromatography system with each size Fibro unit is critical to ensuring optimum performance in terms of buffer usage and elution pool volume. We believe the ready-to-use and the ultrafast purification offered by Fibro PrismA can contribute to increasing productivity of antibody manufacturing processes, especially for clinical and small-batch manufacturing.