We have developed a reliable small-scale model for a monoclonal antibody (mAb) perfusion process using TPP® TubeSpin® Bioreactor tubes and the ReadyToProcess WAVE 25 Rocker bioreactor system.
- The small-scale model with 50 mL TubeSpin cell cultures allowed optimization of process parameters to increase yield in a Chinese Hamster Ovary (CHO) perfusion process. The process parameters investigated were a temperature shift from 37°C to 31°C on different days and at different viable cell densities.
- The temperature shift did not affect the yield. However, operating at a higher viable cell density increased the total product yield and boosted daily product yields by 40%, compared to our standard perfusion process, while achieving similar volumetric productivity.
- The ReadyToProcess WAVE 25 bioreactor demonstrated comparable volumetric productivity of 0.85 g/L/d for 3 days (d). The successful scale-up of the parameters used in the tube-spin experiments confirmed the suitability of the small-scale model.
Introduction
As process intensification is adopted into large scale manufacturing, the responsibility on scale-down models to accurately represent the expanded operating space quickly follows. Perfusion continues to be one such operating mode which lends itself to adding benefit at the manufacturing scale while adding complexity to the small-scale approach.
The purpose of the described study was to develop a reliable small-scale model of our perfusion process in the ReadyToProcess WAVE 25 Rocker bioreactor and WAVE Cellbag 50 L. The aim was to use this small-scale model to screen for process parameters that could increase yield in a perfusion process. Based on literature evidence that temperature downshifts may be favorable for protein productivity by different CHO cells (see reference 1), the process parameters temperature and viable cell density were further investigated in this study. The primary aim of the tube-spin setup was to investigate the effect of a temperature shift from 37°C to 31°C and of identifying the effect of the exact timing of introducing this shift on cell culture productivity.
Materials and methods
Tube-spin experiments for temperature shift evaluation
The effect of temperature shift was investigated on days 2 and 3 (cell density approx. 25–35 million viable cells/milliliter (MVC/mL) and days 4 and 5 (cell density of approx. 50–70 MVC/mL), which represented both early and late time points in the cell culture growth curve. Cell culture of the 37°C tube spin batch terminated at day 12, where culture time for tube spins with temperature shift was extended to 15 d.
For the tube-spin experiments, TPP TubeSpin Bioreactor tubes of 50 mL were used with the powder form of HyClone ActiPro cell culture medium, which was prepared according to the manufacturer’s recommendations. CHO cells were cultured in the TubeSpin Bioreactor tubes in a humified shake incubator. The tubes were fixed in a tube rack inside the incubator on the rocking plate. Other conditions for the tube-spin experiments are shown in Table 1.
On day 0, the cell concentration and viability of the shake flasks were determined using a cell viability analyzer. The required amount of cell suspension to initiate tube spins at 10 MVC/mL initial inoculation density in 5.6 mL working volume was calculated. Tube spins were initiated as duplicates per temperature shift condition and in triplicate for 37°C control condition. The required amounts of cell suspension were transferred into 50 mL conical centrifuge tubes and then the suspension was centrifuged at 150 × g for 7 min. The supernatant (spent media) was removed. The required number of cells was resuspended in fresh HyClone ActiPro cell culture medium and cell suspensions were transferred into tube spins with 5.6 mL working volumes. An extra volume of 0.6 mL was added to the initial culture to correct for the 0.6 mL sampling that was extracted for analyzing cell density, viability, and metabolites.
From day 1 onward, the same experimental procedure was followed except for the sampling volume. A volume of 300 µL of cell culture sample was taken out from the TPP TubeSpin Bioreactor tubes before they were placed back in the incubator. A 200 µL volume of this sample was used for the cell density and viability measurement.
The remaining cell pellet was resuspended in fresh perfusion media. It is important to note that centrifugation at 350 × g for 12 min was used when the cell density reached 50 MVC/mL.
TPP TubeSpin Bioreactor tubes were cultured at 37°C in an incubator. On days 2, 3, 4 and 5, two tubes were moved from 37°C to 31°C and cultured to day 15.
Table 1. CHO cell culture conditions for 50 mL tube-spin experiments
| Parameter |
Setting |
| Incubator agitation rate | 220 rpm |
| Orbital diameter | 50 mm |
| Target inoculation cell concentration | 10 × 106 viable cells/mL |
| Temperature | 37°C and 31°C |
| CO2 incubator concentration | 7.5% |
| Target viability | > 95% |
| Volume of aliquot in TPP TubeSpin Bioreactor tubes | 5 mL |
| Culture medium | Day 0: Hyclone ActiPro |
| Day 1-15: Hyclone ActiPro, Hyclone Cell Boost 1, Hyclone Cell Boost 3 in a volumetric ratio of 1:0.139:0.158 |
Setting up ReadyToProcess WAVE 25 system
ReadyToProcess WAVE 25 system was configured according to Table 2.
Table 2. Characteristics of selected cell culture media and supplements
| Parameter |
Setting |
| Culture working volume | 10 L (day 0) 20 L (day 1 and forward) |
| Dissolved oxygen (DO) control strategy | At seed: 22 rpm, 6° angle DO controlled by automatically increasing the oxygen supply and increase in rpm Interval for settings: 22-35 rpm, 6° to 10° angle |
| Perfusion strategy | Start perfusion when cells have reached ~ 4 MVC/mL Maintain a cell-specific perfusion rate (CSPR) of 20 pL/cells/day Maintain controlled state at 70 MVC/mL by cell bleeding |
| Temperature setpoint | 37°C |
| *pH setpoint | Starting at pH 7.0 on day 0 and lowered to pH 6.8 on day 2 |
| DO setpoint | 40% |
| Gas flow rate at start | 0.50 L/min |
| Target inoculation cell concentration | 1.25 MVC/mL |
| Target viable cell concentration | 70 MVC/mL (controlled state) |
| Target viability | > 95% |
| Culture medium | Expansion batch media: Hyclone ActiPro Perfusion media: Hyclone ActiPro with 2 g/L poloxamer 188, 13.94% CB1 (10% w/v stock solution), 15.80% CB3 (5% w/v stock solution) |
The CHO cells were inoculated at day 0 to a cell density of 1.25 MVC/mL in 10 L. On day 1, the volume was increased to a final volume of 20 L. Perfusion was started on day 3 at a cell density of 4.32 MVC/mL. At day 9, the cell culture had reached 72.5 MVC/mL and the bleed was initiated to keep the cell culture steady at 70 MVC/mL. The culture was terminated on day 12 when a controlled state at 70 MVC/mL had been maintained for 3 d.
Results
Tube-spin experiments
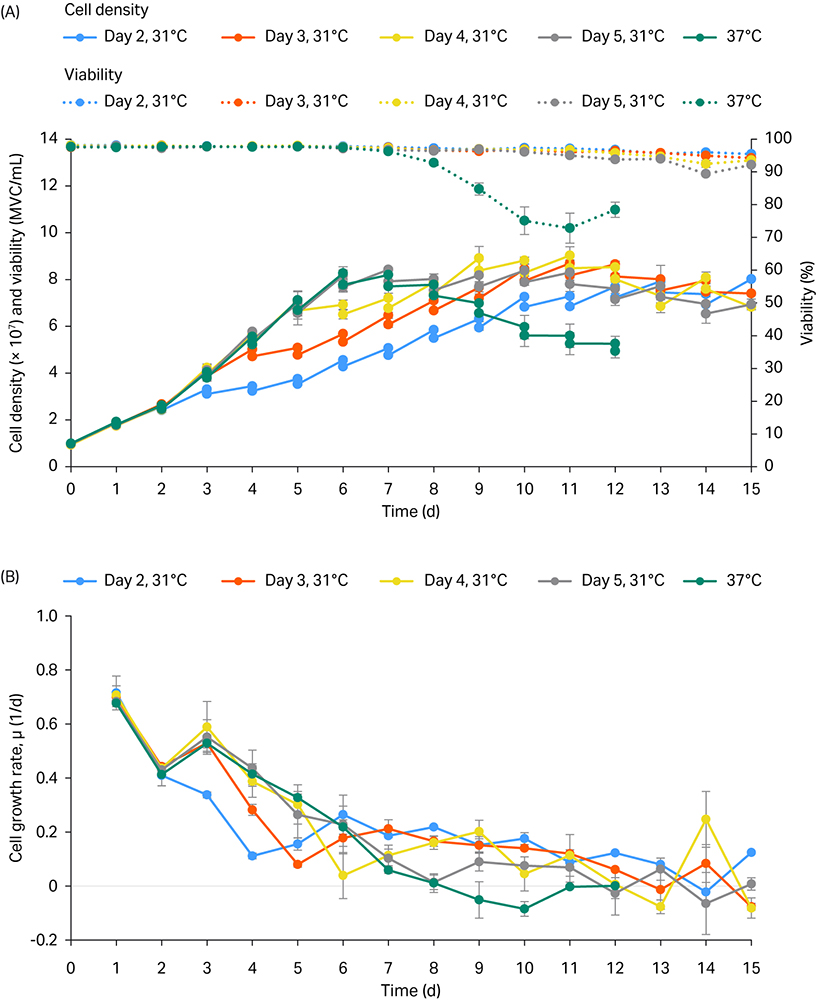
The cell growth and viability profiles are represented in Figure 1 with the averages of duplicate or triplicate tube spins per condition shown.
The culture viability of all the temperature shifted conditions at 31°C was over 90% until day 15 (dashed lines, Fig 1A) and compared favorably with viability at the control temperature of 37°C, which declined after day 7.
Overall, the temperature shift performed on days 2, 3, and 4 caused a decrease in cell growth rate on the following day of temperature shift when compared to the 37°C control (Fig 1B).
Fig 1. Cell density and viability profiles (A) and growth rate profiles (B) of 5 mL tube-spin experiments. Temperature shift (TS) from 37°C to 31°C performed on days 2, 3, 4, and 5.
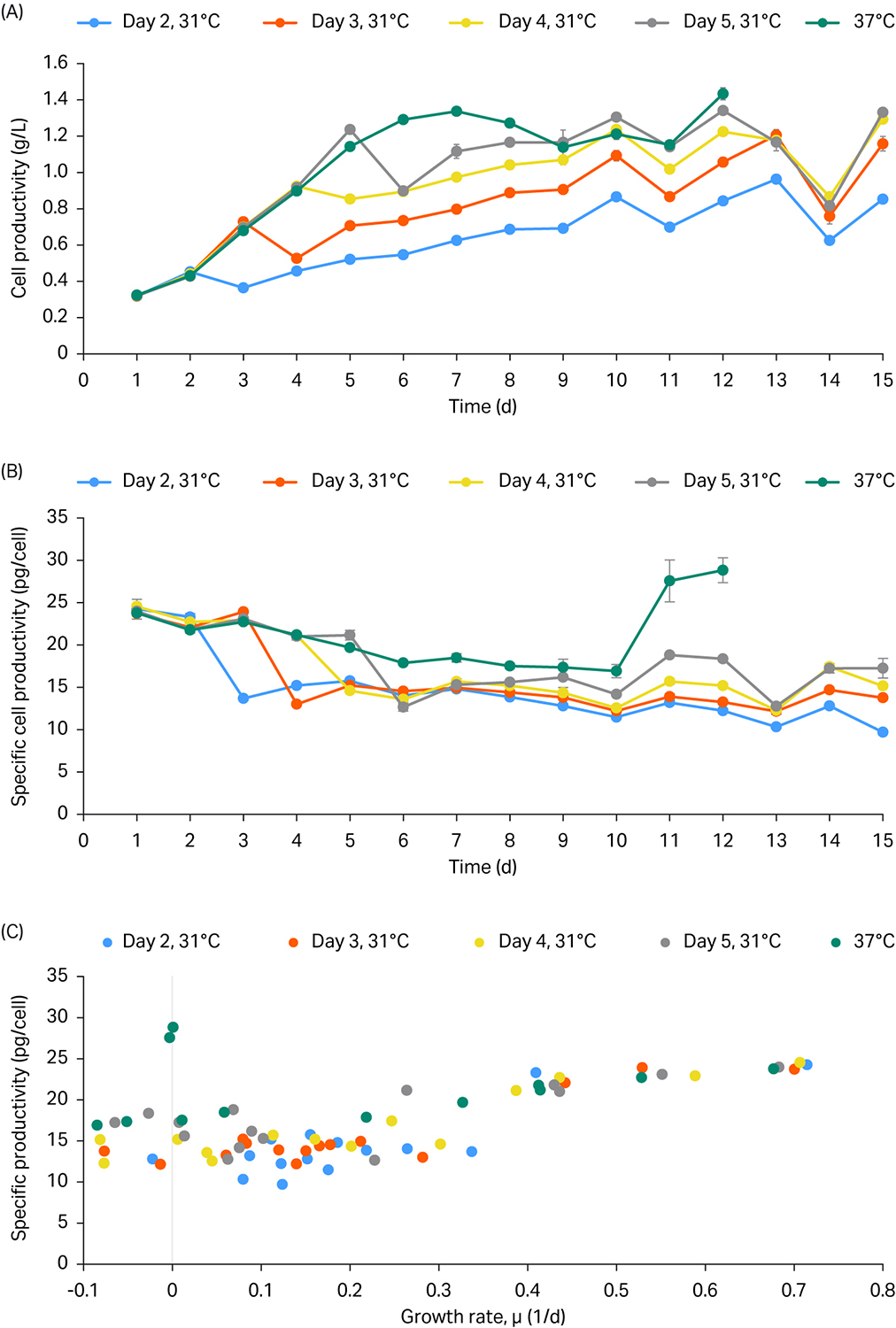
The productivity and cell specific productivity are represented in Figure 2 A and B. Overall, the productivity and cell specific productivity were highest at 37°C control conditions.
Productivity decreased on the day following the temperature shift independent of the temperature shift time point. After the decrease, productivity levels increased daily.
Finally, Figure 2C showed that when the cell growth was above 0.4 to 0.5 (1/d), the cell specific productivity was higher.
Fig 2. Daily productivity (A), specific productivity (B), and specific productivity vs growth rate profile (C). The temperature shift from 37°C to 31°C was performed on days 2, 3, 4, and 5.
These results suggest that productivity was most significantly improved by the late-stage temperature shift on days 4 and 5 compared to the early temperature shift on days 2 and 3. Overall, the temperature shift did not improve productivity and the perfusion culture in ReadyToProcess WAVE 25 was therefore run at 37 C.
ReadyToProcess WAVE 25 cell culture
Cell viability was above 98% throughout the entire perfusion process. Cell density was kept at 70 MVC/mL for 3 d. Cell bleeding was manually adjusted for and tuned twice per day after each sampling and worked well during days 9–12. No cells were observed in the perfusate (data not shown), indicating that the filter was intact during the whole perfusion process.
ReadyToProcess WAVE 25 productivity
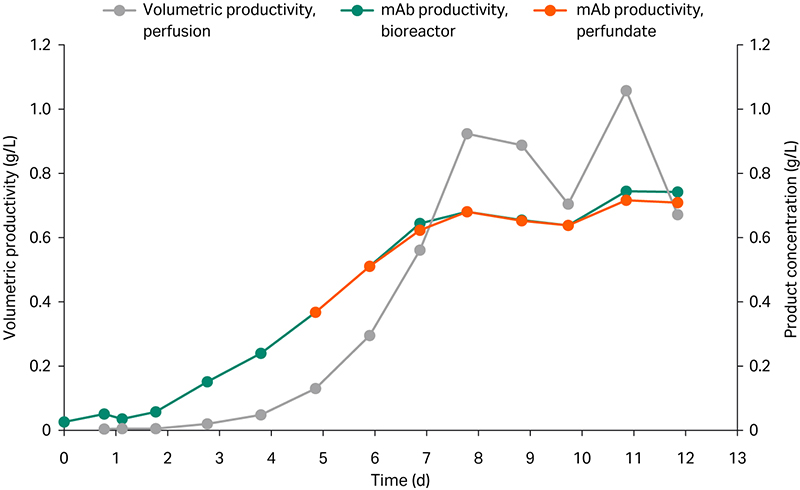
Product titer increased over time, reaching approximately 0.7 g/L during controlled state. No significant difference in titer was observed between the bioreactor and the perfusate after the filter (Fig 3), demonstrating no retention of product due to filter fouling.
Taking the dilution into account, the volumetric productivity reaches above 1.0 g/L/d at one point but fluctuated between 0.7 and 0.9 g/L/d, mainly due to manual bleeding interventions (Fig 3).
Fig 3. Volumetric mAb productivity and product concentration in ReadyToProcessWAVE 25 bioreactor and perfusate, respectively.
Conclusions
A small-scale model for the perfusion process was established using tube-spin experiments. This process was then scaled-up, and run in a 50 L WAVE Cellbag bioreactor for a total of 12 d.
- Daily harvest samples were collected at controlled-state conditions of 70 MVC/mL, with an average volume productivity of 0.85 g/L/d for 3 d.
- We observed comparable volumetric productivity when scaling up the tube-spin experiments to WAVE 25 bioreactor. This reinforced the design of this setup as a suitable small-scale model for perfusion process development regarding cell-specific perfusion rate and cell density at controlled state.
- In summary, the temperature shift did not affect yield; on the other hand, operating at a higher viable cell density increased the total product yield by 40%.
Products used
| Product | ID |
| ReadyToProcess WAVE 25 Rocker | 28988000 |
| HyClone ActiPro powder medium | |
| HyClone Cell Boost 1 powder | SH30584.02, 30584.04 |
| HyClone Cell Boost 3 powder | SH30825.03 |
| WAVE Cellbag 20 L Perfusion Cellbag BioClear 11 film | CB0020L11-34 |
| Wave Cellbag 50 L Perfusion Cellbag BioClear 11 film | CB0050L11-34 |
| ReadyCircuit Disposable Assembly 50 L | 12410228 |
| ReadyCircuit Disposable Assembly 20 L | 12410224 |
| ReadyFilter NFF Capsule Assembly | 12410094 |
| ReadyToProcess Hollow fiber cartridge, 0.65 µm, 950 cm2 | RTPCFP-6-D-4X2MS |
| SciLog pressure sensor | 28979471 |