The requirements for speed and economy are climbing in the biopharma industry. Go/no go decisions are being made earlier, and products need to be brought to market faster and at a better price point. These needs translate into increasing pressure to quickly create processes that are cost-effective at manufacturing scale. Gains in protein productivity can cumulatively make a real positive impact on your process economy.
When evaluating media with the goal of increasing titers, it is important to ensure that protein quality is not negatively impacted. So how can this process improvement be performed reliably? It makes sense to use a Design of Experiment (DoE) and empirical approach. Here is an example of how to do it.
Case study: improving both yield and quality
When developing a cell culture process, you might be interested in improving peak viable cell density (PVCD), product levels, product quality, or other attributes. In some cases you might be able to increase more than one of these. Regardless of the goals, choosing a robust methodology for cell culture medium evaluation will help you to quickly identify and test relevant parameters.
Let's illustrate the process by looking at a practical example.
The issues
During biosimilar development results showed:
- Low peak viable cell density (PVCD) and product levels
- Low product quality (low levels of the tri- and tetra-sialylated glycans that dictate bioreactivity)
The goals
Key to solving the issues was finding ways to:
- Improve productivity
- Enhance tri- and tetra-sialylated glycan
- Improve tri-antennary and tetra-antennary glycan structures
The process
The process started with the idea of designing a mixture to achieve an optimized formulation.
1. Seven basal media were selected from a library of reference formulations as the best potential matches for the specific CHO cell clone.
2. Growth curves and viability profiles were evaluated.
3. A DoE simplex lattice mixture design study using the top four prototype media – in 28 conditions including a control – was implemented. Results were collected and compared with the control mixture.
4. Prototype mixtures were evaluated for PVCD, integral viable cell area (IVCA), product levels, and product quality.
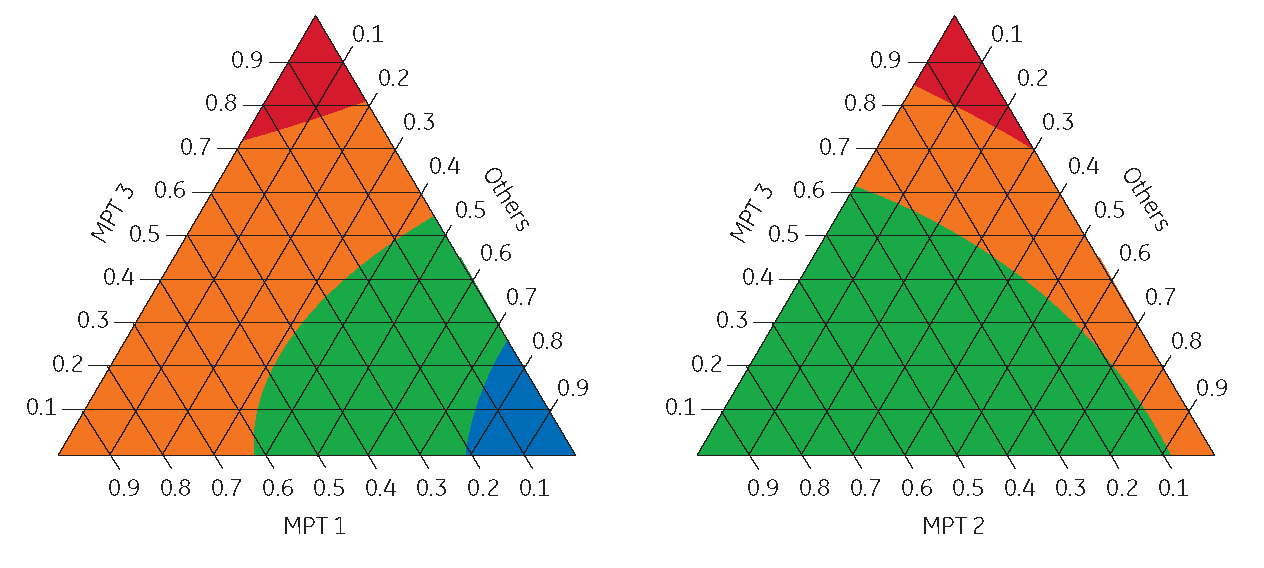
5. Ternary plots of DoE mixture design prototype conditions were created. “Hot Spots” (red) on these plots showed that mixtures higher in media prototype 3 (MPT3) from the initial screening yielded higher product levels.
Mixtures higher in medium prototype 3 (MPT3) from the initial screening yielded higher:
- product levels (shown)
- IVCA
- PVCD
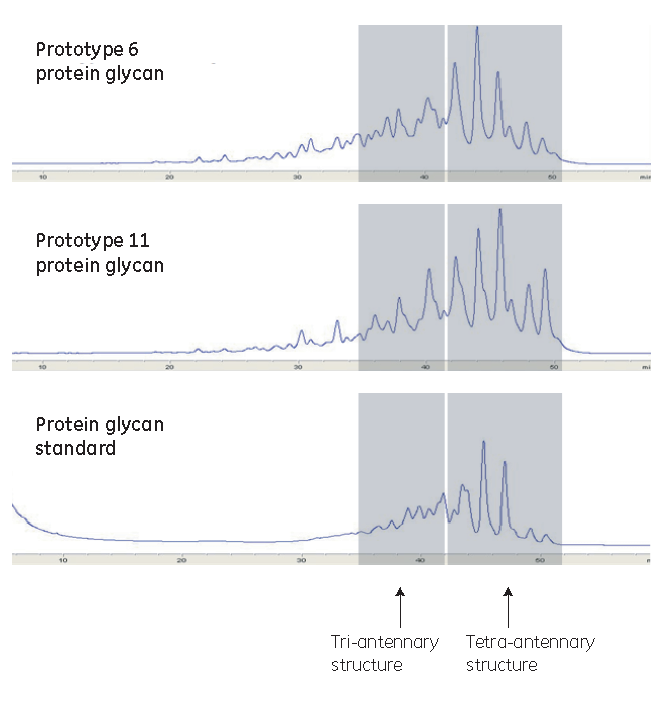
6. Attention was then turned to the product quality. Two types of HPLC analysis were used to compare the quality of the proteins. Compared with the control medium, three prototypes provided improvements in both desired protein quality attributes. Two prototypes had similar formulations, so one was excluded.
Normal-phase HPLC analysis
7. The results from the two remaining candidates were then compared and evaluated to make the final selection.
The results
Prototype 6 exhibited a PVCD at 219%, an IVCA at 276%, and peak product levels at 192% of the control medium, in addition to quality improvements in sialylation and antennary structures.
Ultimately, this prototype was chosen for cell banking and further studies because of slightly higher product levels and quality than the remaining prototype.
Benefit from medium optimization
With these results, it is clear that finding the right medium offers real opportunities for optimization of quantity and quality. It could be worth investing in creating a customized medium to achieve longer-term results, particularly when you get to process-scale production.
From choosing the right potential basal media to creating a well-formulated design study, applying a well thought-through approach is essential. We can help by providing you access to our formulary library, and we can work alongside you to find the optimized medium for your process.
- Improve cell culture performance, productivity, and product quality – learn more about our custom designed media services to meet your specific production goals.
- Discover how employing DoE in custom medium development can increase protein quantity and quality – download the poster.
- Learn about a rapid and efficient approach to media and feed optimization to increase CHO antibody production – download the poster.
- Solve your cell culture scale-up challenges with single-use technology and support from skilled technical experts at Cytiva – read the white paper.
- Explore how Fast Trak Analytical services provides support and troubleshooting for development projects. One service, spent medium analysis, can identify which components are depleted or in excess over the course of the culture. Based on the results, you can adjust the various components to reflect their actual use – view the brochure.
- When small volumes of customized cell culture products are required quickly, consider RRP services. RRP can replicate standard manufacturing practices for customized quick-turnaround media, supplements, and reagents packaged in bottles or single-use containers – view the brochure.