Rapidly address healthcare needs with adaptable biomanufacturing options
In biomanufacturing, the only certainty is risk and the only constant is change. Future-proofing any capacity expansion to allow for the unknown product or technology is challenging. Location, personnel, timing, process, and planning are critical success factors. KUBio™ modular facilities are designed to reduce project timelines from years to months anywhere in the world. Explore your options based on your workflows and locations. Replicate, scale out, or scale up with Cytiva’s in-depth expertise, proven solutions, and end-to-end support.
REQUEST A SAMPLE LAYOUT
REQUEST A SAMPLE LAYOUT
- What is the lead time for a KUBio™ facility?
- 17-20 months
- How much land do I need for a KUBio™ facility?
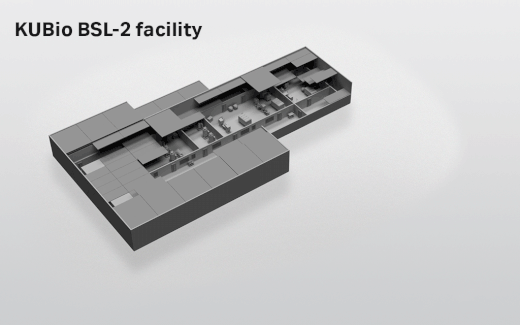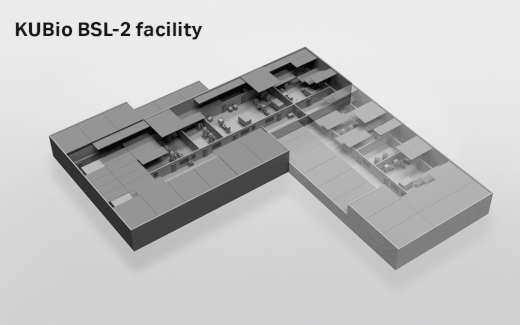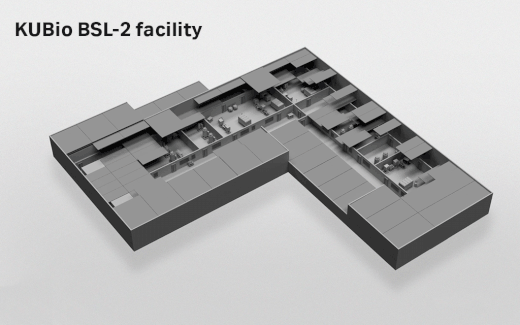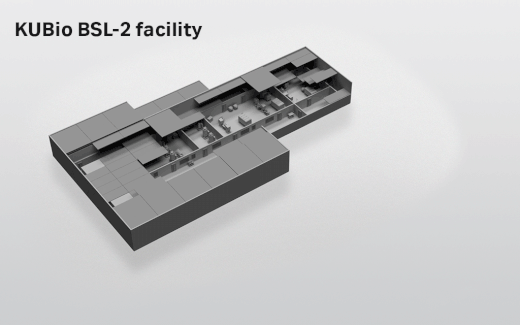
- Depending on which facility you select, the actual footprint ranges from 1800 square meters (KUBio™ BSL-1) up to 3800 square meters (KUBio™ BSL-2 fully expanded).
- What is the production scale in a KUBio™ facility?
- KUBio™ facility batches range from 2 x 500 L to 6 x 2000 L scale.
- What products can I make in a KUBio™ facility?
- KUBio™ BSL-1 facilities can accommodate mAbs, mRNA, and pDNA.
- KUBio™ BSL-2 facilities are designed for controlled workflows typically requested for viral vectors and vaccine manufacturing.
- What is the difference between a KUBio™ facility and a KUBio™ box?
- A KUBio™ modular facility is a stand-alone manufacturing facility including generation of clean utilities and treatment of effluents (solid, liquid, and airborne). A KUBio™ box modular environment is placed inside your infrastructure and connects to your utilities.
- What is the relation of the FlexFactory™ platform to a KUBio™ facility?
- A KUBio™ modular facility is the building while the FlexFactory™ biomanufacturing platform supports the manufacturing equipment that is placed inside. Both are designed for product workflows and scale. A KUBio™ modular facility always includes the FlexFactory™ platform.
- Can Cytiva help design my manufacturing workflow?
- Yes! Process design is the key to a successful project. We have gained expertise across a wide array of successful projects.
- What kinds of automation can I use in a KUBio™ facility?
- Cytiva Figurate™ automation controls the equipment and can be tailored to your design requirements. We support a variety of automation platforms including DeltaV™, PlantPAx™, and UNICORN™.
- Who prepares the site for the KUBio™ facility?
- The clients coordinate and prepare the site and site infrastructure for the KUBio™ modules, which includes the foundation and the supply of technical utilities like steam, water, power, and sewage.
- Who coordinates the shipment and installation of the KUBio™ modules?
- Cytiva coordinates the shipment and installation of the KUBio™ modules as well as the FlexFactory™ manufacturing line components.
- What support does Cytiva provide for my manufacturing capacity expansion?
- The KUBio™ facility is a turnkey solution, pre-designed and ready for engineering runs directly after handover of a qualified facility and process. Cytiva assists in designing the FlexFactory™ manufacturing platform to fit any future plans to scale your production capacity up or out.
- How can Cytiva help us keep our projects moving forward while we’re waiting for the KUBio™ to be completed?
- You can collaborate with our Fast Trak™ scientists to optimize and streamline your manufacturing in parallel with the KUBio™ facility development. The Fast Trak™ suite of services includes training and education, process development, CDMO manufacturing, and process validation. Our local Fast Trak™ service teams support process transfer to emerging regions, source local raw materials, and understand local government and regulatory requirements.
- Where can the KUBio™ BSL-2 facilities be built?
- The KUBio™ can be built anywhere around the world.
- Is the KUBio™ BSL-1 expandable?
- We do not have an expandable BSL-1 facility. However, KUBio™ for BSL-1 can be used for a production bioreactor configuration from 2 x 500 L up to 4 x 2000 L including required seed bioreactors.
As an option, KUBio™ BSL-1 can be expanded to include up to 6 x 2000 L production bioreactors, supported by one downstream (purification) train.
- We do not have an expandable BSL-1 facility. However, KUBio™ for BSL-1 can be used for a production bioreactor configuration from 2 x 500 L up to 4 x 2000 L including required seed bioreactors.
- What is the build time for Phase I and II? How much time can be left between Phase I and II builds? Do I need to commit to both Phases from the start?
- Depending on country-specific laws and regulations, Phase I will be built in 15-18 months. The extension with Phase II will be built in 11-16 months with only a short interruption of on-going operations in Phase I when connecting the two building parts.
There is no need to commit for both phases from the beginning, but the facility is prepared for the extension independently whether or not it will be implemented in the future.
- Depending on country-specific laws and regulations, Phase I will be built in 15-18 months. The extension with Phase II will be built in 11-16 months with only a short interruption of on-going operations in Phase I when connecting the two building parts.
- Does the expansion from Phase I to II affect the use of the existing structure, or can I continue manufacturing while the building is progressing?
- The facility can keep operating during the expansion phase. Operational interruption will happen during the time when the original facility and the facility expansion are connected and revalidated, but this time is minimized as much as possible.