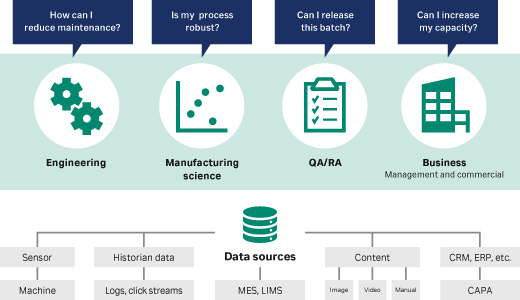
Reduce risk for a robust process
Your risk is our concern. That’s why we test and monitor hundreds of raw materials and identify the high-risk materials with the biggest impact on product quality.
Webinar: Control process variability through analytics
Sourcing
Global raw material sourcing, specifications, and testing aligned across all cell culture manufacturing sites.
Global raw material sourcing, specifications, and testing aligned across all cell culture manufacturing sites.
Testing
Testing program for critical raw material components. Tests include: HPLC, UHPLC, ICP-MS, and more.
Testing program for critical raw material components. Tests include: HPLC, UHPLC, ICP-MS, and more.
Monitoring
Proactive approach to monitor and address issues.
Proactive approach to monitor and address issues.
How do you trace each raw material back to its original source?
- We actively work with our suppliers to trace raw materials back to their sources by rigorously tracking basic supplier information and completing supplier maps and supply chain analyses. We ensure our suppliers are using the same materials, processes, and equipment to produce incoming raw materials.
What are some common trace impurities in your raw materials and where do they come from?
- Cell culture media products are highly complex and can be comprised of up to 100 different components. Some of these components contain trace impurities, such as metals, reaction intermediates, and residual solvents. Many of these trace impurities originate from raw material sources (mining, extraction, etc…), manufacturing inadequacies, or storage and handling. The contamination profile for each raw material can vary on a lot-to-lot or vendor-to-vendor basis. This inherent raw material variability can cause inconsistent media attributes between batches, even when the same suppliers and processes are used in production. We make sure that all cell culture media products meet our stringent performance and consistency standards by assessing every batch of raw materials for impurities.
How is cell culture media performance affected by raw material variation?
- Raw material inconsistencies within a biomanufacturing process can cause production variability for pharmaceutical customers. Even the slightest impurity can influence the ability of cell culture media to support cell growth in later stages of development or therapy production. Unpredictable performance can potentially affect drug quality and predictable yields, reduce manufacturing uptime, increase overall costs, decrease customer confidence, and negatively impact patients. One of the most meaningful paradigm shifts in the biopharma industry in recent times has been the realization among therapy researchers and producers that close control of raw material parameters is essential in securing repeatable process performance, from the research lab to process development and full-scale production.