Cell and gene therapies (CGTs) offer the potential to transform clinical care, evident by increasingly diverse treatment applications and the launch of more products on a global scale. Since patient cells are the foundation of these lifesaving therapies, CGT supply chains present unique challenges not faced in the development, manufacturing, and transport of biologics. Cells require special care and consideration, such as strict temperature controls and the ability to track and verify chain of custody from collection to final formulation.
Many of the tools and solutions currently used to address these challenges rely on laboratory-based methods that are manual and time-consuming and introduce a considerable level of risk. To ensure the industry can realize the potential of CGTs, we must develop improved solutions that reliably support the control and transport of critical CGT raw materials and products.
Temperature control for CAR-T cell therapies
CGTs represent the fastest-growing segment in the regenerative medicine market, with the majority of success thus far coming from CAR T-cell formulations.1,2 This type of immunotherapy, used to treat certain blood cancers and a potential option for other cancer types, has a success rate of about 30% to 40% in achieving remission without additional treatment.3 Driving the immense impact of CAR-T therapy on clinical care is the fact that many of the patients who benefit from these treatments have exhausted all other options for survival. With the potential to change prognoses and some notable CAR-T approvals by the FDA in recent years,4 there is a growing demand for these medicines. To answer this call, CAR-T manufacturers need effective and efficient temperature control strategies.
Acquisition of starting materials for CAR-T therapies begins at the clinic, where a patient provides an apheresis sample. Next, several processing steps prepare the sample for transport, including the controlled freezing of extracted cells to cryogenic temperatures (below -120°C). Once frozen, the cryogenic temperature must be maintained during shipment to the lab or manufacturing center to prevent deterioration. When processing is complete, the final product is typically cryopreserved again for shipment back to the patient.
Cryopreservation is an accepted method for temperature control of cellular products, but proper execution has traditionally required special space, equipment, and education. To work with cryopreserved materials, you would typically need a large tank of liquid nitrogen onsite, dedicated storage facilities, and shipping containers that can hold liquid nitrogen during transport without the risk of spills or leaks. Anyone retrieving cryopreserved materials or transporting large shipping containers will also have to receive specialized training on handling liquid nitrogen.
To overcome these challenges and requirements, CAR-T cell therapy developers and manufacturers need equipment specifically designed to sustain cryogenic temperatures without liquid nitrogen. Cytiva’s VIA Capsule™ system — comprised of a shipper unit, an integrated smart monitor, and a cryocooler system — uses electricity to cool temperatures down to the target range. The system can also track and verify chain of custody between multiple stakeholders, from collection to delivery, addressing another important factor in protecting raw materials critical to CGT production.
Managing chain of custody for CGTs
Autologous CGTs use a patient’s own cellular material for treatment. Knowing where these materials are at all times throughout the complex workflow is essential to ensuring quality, efficacy, and delivery back to the right patient.
Starting materials could be collected at a hospital or clinic before being passed on to a processing laboratory for target cell isolation and cryopreservation. The resulting cellular product is then packed for shipping in a container provided by the clinic, the courier, or a third party. If the CGT manufacturer is in a different country than the patient, a courier takes the container with the starting material to an airport. Once there, an airline or grounds crew moves the material to an aircraft and, upon arrival, hands it off to another airline or grounds crew. A second courier then takes the starting material to the manufacturing facility to convert it into a patient-specific treatment product. When processing is complete, this complex journey must be reversed to get the therapy back to the patient.
Today, CGT chain of custody is managed by placing labels with a unique identifier on the product as it moves from point to point. A paper-based process is used to verify when the product reaches each of its handover points. Transparency during this process is critical not just for tracking but also for maintaining the timeline necessary to prevent a lost batch. In traditional biologics manufacturing, product loss is costly, but other batches are still being produced to help meet patient needs. With autologous therapies, any errors or issues during transport can have devastating consequences to the patient waiting on treatment. A reliable digital system and software solution is needed to track starting materials from the moment they leave the clinic to the second the final therapy is delivered back to the patient.
In addition to offering a liquid nitrogen-free shipping option, the VIA Capsule™ system comes equipped to work with Cytiva’s Chronicle™ automation software. Chronicle™ software provides a unified digital platform to monitor manufacturing operations and supply chain logistics for cell therapy facilities. These innovative tools can overcome the industry’s biggest challenges with acquiring cell therapy starting materials and managing the associated supply chain. A collaboration between the Advanced Therapy Treatment Centres (ATTC), several industry partners, and the public sector supported the design of these solutions.
ATTC cell therapy logistics program
In 2018, the Industrial Strategy Challenge Fund awarded 30 million pounds to support the creation of the three centres — joining hospitals, advanced therapy medicinal product (ATMP) manufacturers, and equipment, software, and service providers — within the United Kingdom (UK) to make up the ATTC network. The ATTC and other specialist centres aim to establish systems to support CGT clinical supply, clinical adoption, data acquisition, and reimbursement. The network helps companies and NHS partners quickly develop and demonstrate their offering, while driving expansion into international markets. By coordinating within the ATTC network, the UK’s National Health Service and industry organizations can work together to create clinical pathways for the delivery of CGT products.
As part of these efforts, the ATTC conducted a study to develop and test an integrated cell therapy logistics network of manufacturers, couriers, distribution centres, and clinical trial sites using Cytiva’s VIA Capsule™ system and Chronicle™ software. In summary, the SAMPLE (Standard Approach to ATMP Tissue Collection) program focused on the acquisition of starting material for CGT manufacturing workflows, with a vision to:
- Reduce ATMP variability/failure (caused by poorly/undefined quality attributes of samples exacerbated through practices, devices, and settings)
- Streamline delivery and develop a robust and connected supply chain by defining the process map for scheduling collections and coordinating logistics between providers and sites
- Identify supply chain weaknesses (i.e., key equipment, trained staff, slots) for ATMP starting material collections
- Test closed-system processing and cryopreservation at collection sites to enable industrialization of ATMPs through starting material standardization for manufacture
The entirety of this study used Chronicle™ software to examine and enable detailed data collection and monitoring during a long and complex CGT journey. To start, scientists in Galway, Ireland could scan multiple sample-specific elements, including the shipping instrument, to confirm the product was packed into the correct VIA Capsule™ system. The shipping system was then sent to Orbsen™ Therapeutics manufacturing center at the University of Birmingham in the UK, where eSOPS were used to execute mock unloads at critical conditions. Shipment data were logged into Chronicle™ software again to maintain the chain of custody throughout travel, which included several sites in London. Remote monitoring within the Chronicle™ platform was used to track location and product conditions as the shipment moved through the logistics chain from partner to partner (Fig 1). The cellular product was then returned to Orbsen Therapeutics for the final assessment using two different viability assays. Orbsen conducted tests that clearly showed no significant difference in cell viability between sample cells shipped and monitored through a complex journey and control cells kept at the storage facility.5
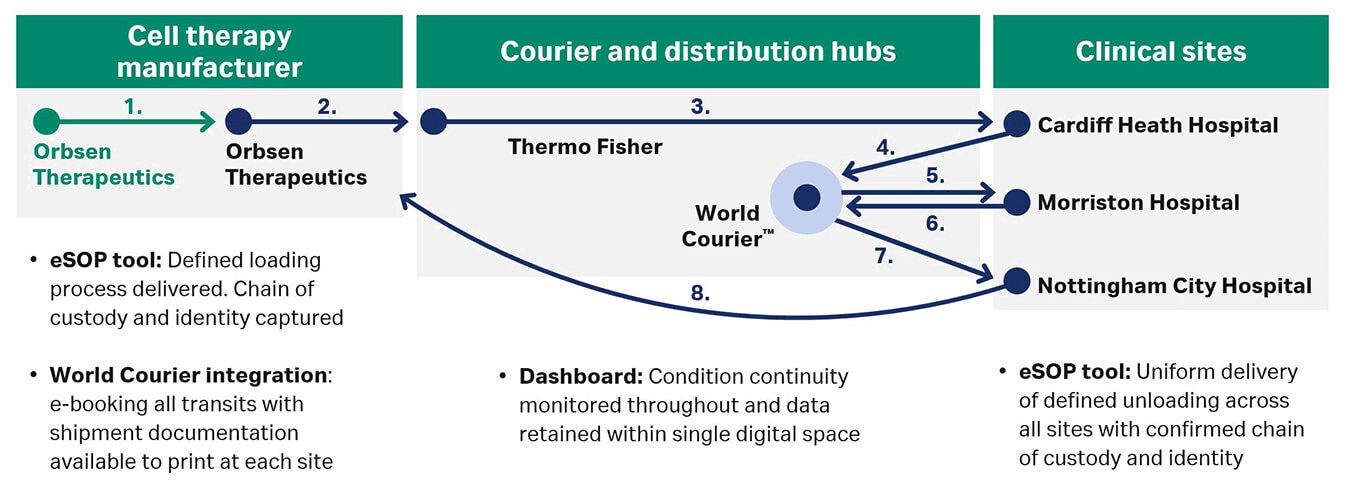
Fig 1. Logistics chain in ATTC SAMPLE study.5
The growing CGT market, while ripe with opportunity, also faces a wide range of challenges. As the industry explores new technologies and methods to increase efficiency and lower costs of manufacturing, it must also secure critical raw materials throughout their journey from the patient to production and back. Automated solutions and collaborative efforts, such as those demonstrated by the ATTC network, serve as key components in driving vital changes that will lead to the production of CGTs at the scale necessary to meet patient and market demand.
References
- Grand View Research. Gene Therapy Market Size, Share & Trends Analysis, Report Overview. Published Feb 2021. Accessed 22 March 2021.
- GlobeNewswire. The global cell and gene therapy market by revenue is expected to grow at a CAGR of over 30.90% during the period 2019–2025. Published 4 Aug 2020. Accessed 22 March 2021.
- Bartosch, J. UChicago Medicine. Three years after CAR T-cell therapy for lymphoma, patient still cancer-free. Published 17 October 2019. Accessed 22 March 2021.
- UPMC Hillman Cancer Center. FDA-approved CAR T-cell Therapies . Accessed 22 March 2021.
- Permission to publish ATTC SAMPLE study findings has been granted by Orbsen. Data available upon request.