Apheresis in the development of cellular immunotherapies
Recent scientific advances have led to an improved understanding of cancer and the development of personalized medicines, such as immunotherapies.
T cell receptor (TCR)-based treatments, such CAR-T cell therapy, have become a focus of research and could eventually play an important role in standard of care for cancer. To date, the FDA limits the clinical use of CAR-T cell therapy to certain types of leukemia and lymphoma at approved cancer centers. The success of Kymriah™ (Novartis) and Yescarta™ (Gilead/Kite) immunotherapies, however, has set the stage for similar new and effective strategies to potentially eliminate resistant tumors.
Cells therapies are developed using autologous or allogeneic genetically modified cells. Making these treatments — and the processes used to create them — adaptable to many patients remains a key challenge in standardizing immunotherapies, due to inherent and significant source product variability.
Peripheral blood collection with apheresis processing is a preferred method for obtaining patient source material for cell therapies, as it is minimally invasive and results in the collection of a large number of cells. Any biological products collected from apheresis that have increased variability or decreased quality can negatively affect subsequent processing, making this step crucial to the success of the therapy itself. Cell therapy manufacturers might need to employ subprocesses, decision matrices, and more complex analytics to accommodate incoming product variability. Moreover, once transition to commercialization begins, the volume of patients and number of collection sites both increase, as does the need to focus on the robustness of the supply chain and starting material.
This article contains a collection of resources and information about apheresis products and their characteristics, manner of collection, and processing. The aim of this report is to offer guidance and best practices for apheresis collection and obtention of enriched product for cell therapies.
How apheresis works
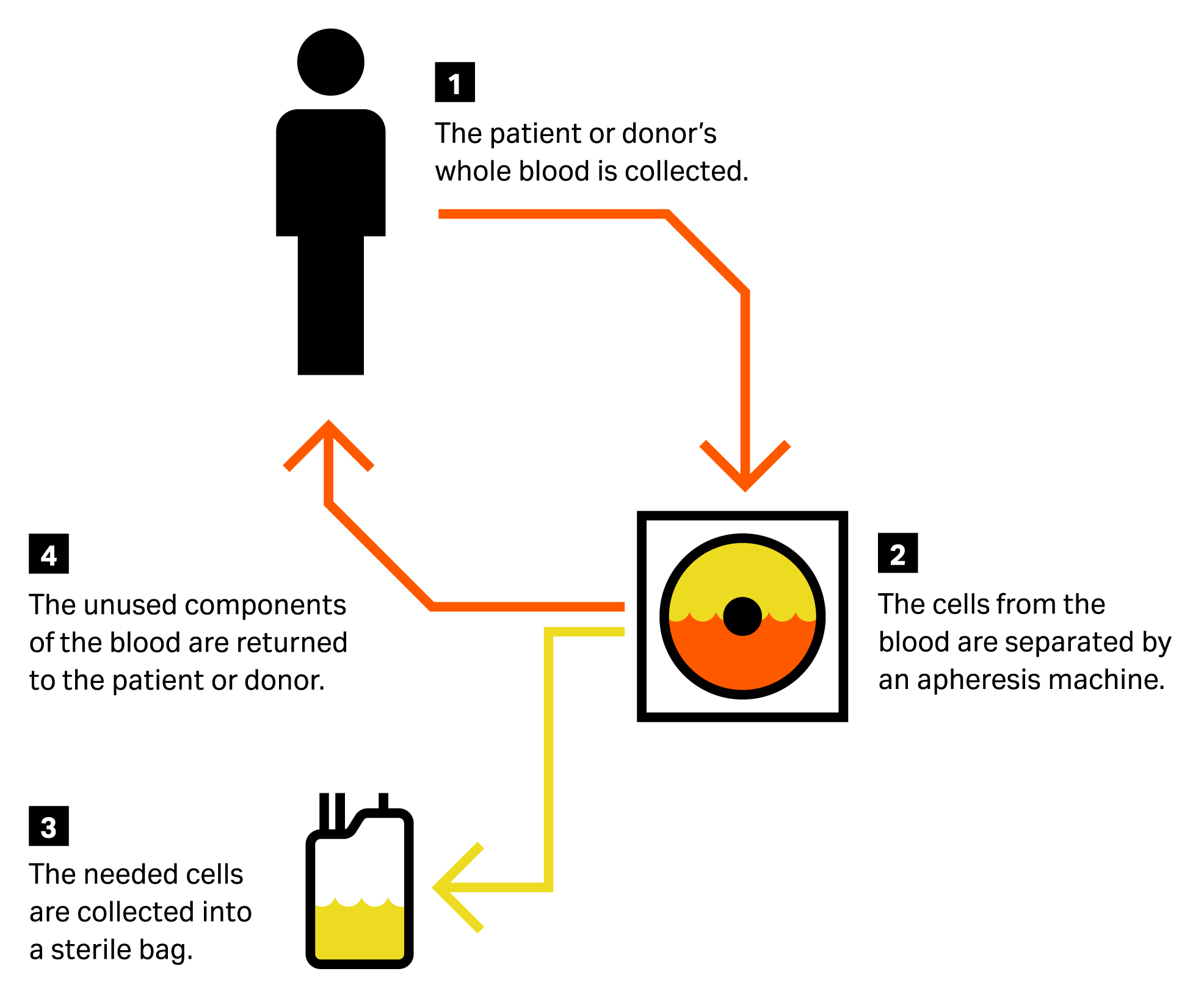
Apheresis refers to a series of procedures performed on patients to remove and divide peripheral blood into components. An apheresis collection device is used to collect and separate certain components — such as plasma, platelets, and white blood cells — and then reinfuse the remainder back into the patient (Fig 1). Different types of apheresis include erythrocytapheresis (retention of red blood cells), leukapheresis (retention of leukocytes), plasmapheresis (retention of plasma), and plateletpheresis (retention of platelets).
Transfusion centers for regenerative medicine and cell processing use leukapheresis to isolate leukocytes (lymphocytes, monocytes, stem cells, etc.) that can be used to bioengineer a patient-specific cell therapy (autologous treatment) ex vivo.
Fig 1. Apheresis process illustration.
We generally distinguish between two types of leukapheresis: mobilized and non-mobilized.
The objective of mobilized apheresis is to obtain hematopoietic stem cells (HSCs). With mobilized apheresis, patients/donors need to be conditioned prior to apheresis collection to move HSCs from bone marrow to peripheral blood.
With non-mobilized apheresis, patients/donors do not undergo pre-conditioning. The goal of this type of apheresis is to obtain differentiated cells, such as B and T lymphocytes or monocytes. This patient-specific source product provides the starting material for the development of immunotherapies.
The many sources of variability in apheresis
Developing a process that is flexible and adaptable to all patients, despite source product variability, is a big challenge in immunotherapy manufacturing. There are many variables related to the apheresis process that must be accounted for, including:
- Patient-specific variables, such as age, ethnicity, and diagnosis
- The performance of the apheresis collection device used
- Shipping and storages conditions, such as buffers and temperatures
- Blood composition variability within post-processed apheresis product
Some of these variables can be standardized, while others are patient specific and might remain uncontrolled. Below, we share process control strategies that will minimize inherent patient variability, deliver more predictable cell therapy products, and achieve more consistent treatment safety and efficacy profiles for optimal patient care.
Standardizing apheresis to reduce variability
Many parameters can affect the quality of materials used to manufacture cell therapies and influence the final product. These parameters include unique donor characteristics, the apheresis collection device, and shipment and storage conditions.1,2 Where control is possible, keeping these factors consistent can help reduce process and product variability.
Shipment and storage conditions
After apheresis processing, the cell product material must be stored and shipped from the central processing center. Identifying optimal conditions for cell therapy storage and transport between sites is key to maintaining high cell viability. In past years, extensive studies were published involving healthy volunteers who underwent non-mobilized mononuclear cell (MNC) collection by apheresis to determine optimal storage conditions.3
These studies revealed the following:
- Refrigerated storage (4°C to 8°C) preserved viability greater than room temperature (RT) storage (20°C to 22°C), especially for products with a higher cell concentration.
- RT maintenance with a high cell concentration was associated with a relative loss of white blood cells.
- Platelet activation was observed in many apheresis procedures, inducing aggregation of monocytes with platelets.
In summary, it is recommended to use the same collection device across all procedures and to store and ship apheresis product at 4°C to best preserve and maintain cell viability, cell number, and phenotype.
Increase manageability of highly variable apheresis parameters
Cell content heterogeneity and MNC enrichment
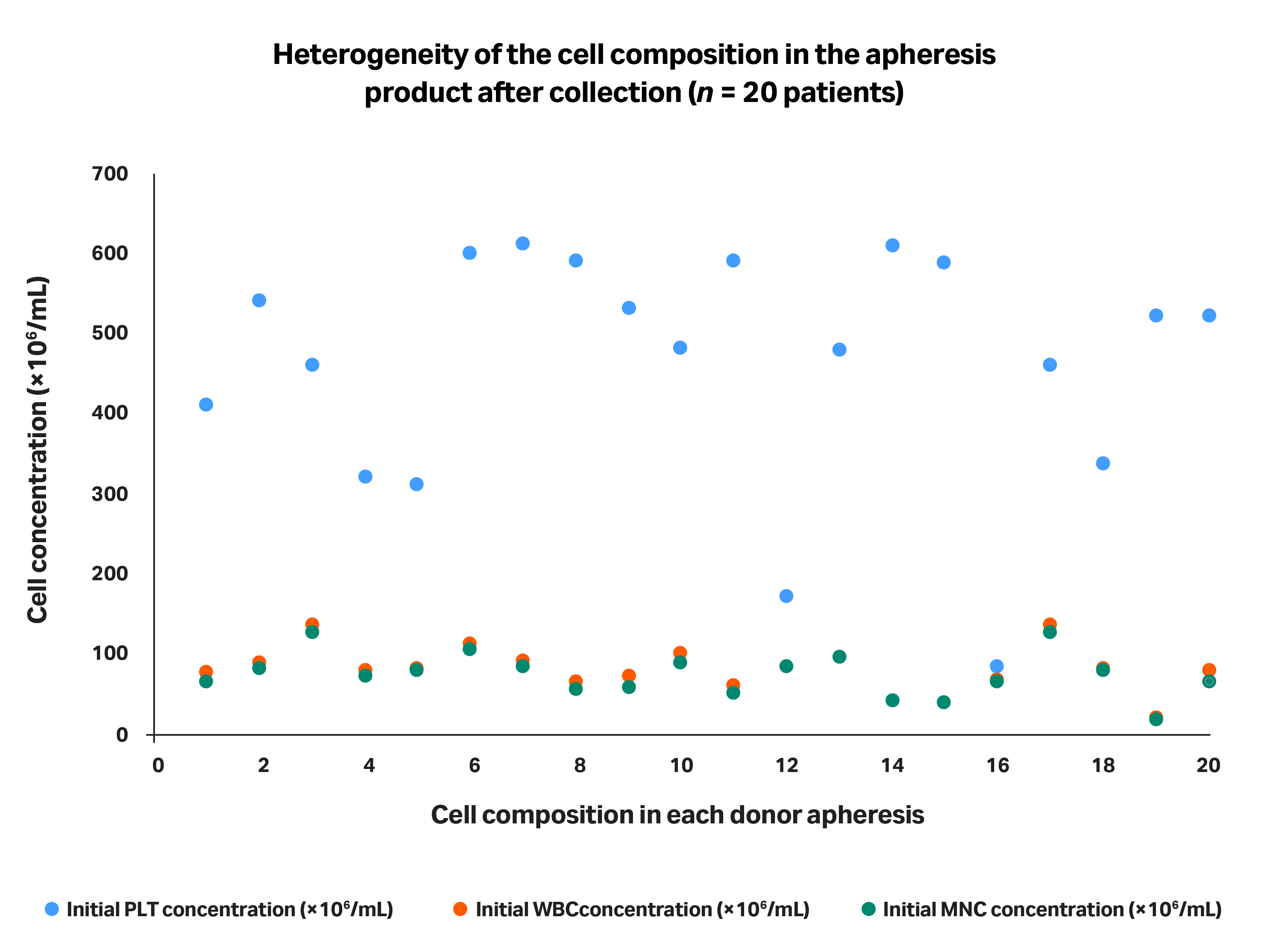
Figure 2 highlights the heterogeneity of the cell composition in several apheresis products after they were processed using same collection device.
Fig 2. Representation of apheresis product heterogeneity in terms of cell composition after they were processed using the same collection device. Each of the 20 patients’ data points are grouped in a single column.
In order to obtain the mononuclear fraction from blood following apheresis collection, a second cell processing step is required to remove platelets, red blood cells (RBCs), and granulocytes, enriching the product in MNCs. The goal of this step is to reduce the heterogeneity induced by these cell populations and minimize composition differences due to patient-to-patient variability. This step also serves to maximize the yield of the desired MNC population. Beginning your ex vivo manufacturing process with a higher starting cell number can facilitate robustness during cell phenotype isolation and accelerate the time necessary for the cell expansion step, allowing you to reach the desired final dose more quickly.
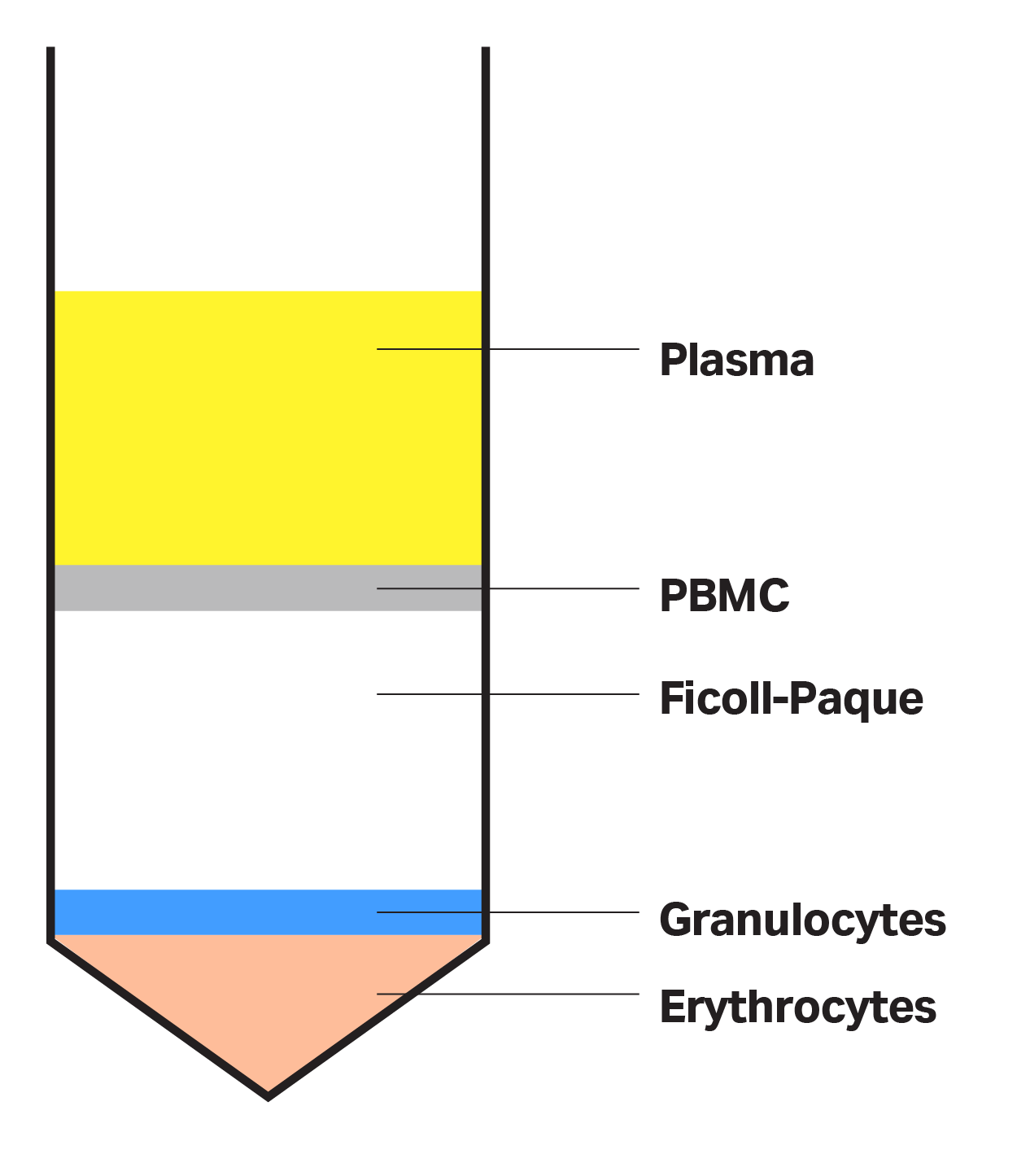
During this enrichment phase, mononuclear cells, such as monocytes and lymphocytes, are isolated from platelets, plasma, RBCs, and granulocytes (GRCs) based on varying component densities. You can achieve this separation using density gradient separation media, such as Ficoll-Paque™ media (Cytiva). Ficoll™ media has a specific density (1.077 g/mL) that sits between the density of RBCs and granulocytes on one side, and mononuclear cells, platelets, and plasma on the other. The layer of density gradient medium allows for an effective isolation of the different components following centrifugation, with high density cells, such as RBCs, at the bottom and lower density layers containing plasma and platelets on top (Fig 3).
Fig 3. Ficoll-Paque™ density gradient separation media divides low density blood components from higher density RBCs and granulocytes.
The removal of cells that will not be used for therapy reduces variability and increases standardization. Moreover, these non-needed cells can contribute to negative outcomes when included in immunotherapy formulations.
RBC removal is important because RBC contamination adds a red color to the final cellular therapy — pharmaceutical regulators can see this as an impurity, possibly leading to a non-conform product. Depleting plasma and platelets upstream can also help promote the successful removal of other non-needed cells by curbing platelet activation. Activated platelets can attach to other cells and create aggregates or clumps, altering cell density and affecting the efficiency of the density gradient medium separation. Other factors known to induce platelet activation include high cell concentration, long storage time, absence of calcium chelators, the type of collection device used, and strong mechanical forces from centrifugation.
CASE STUDY: Identify the optimal scenario for MNC enrichment
We have investigated best practices for minimizing the variability of MNC-enriched product obtained from initial apheresis, aiming to standardize the process across patients in order to improve control and predictability when manufacturing immunotherapies.
Historically, the first steps of the MNC enrichment process have consisted of:
- Concentration of the input product
- RBC depletion via density gradient medium
This process can sometimes lead to issues such as:
- Platelets attaching to monocyte (aggregate formation)
- Drop in monocyte viability
- RBC contamination (seen by evaluating the color of the final product)
By correlating the level of RBC contamination (red color) with the characteristics of the initial apheresis unit (cell content repartition), the following assumptions were established:
- RBC contamination could be correlated with the platelet content of the input apheresis product
- Platelet depletion upstream to RBC separation helped avoid platelet activation, which caused aggregates and impacted the efficiency of RBC separation
From this experience, we propose that platelet count plays an important role in the first steps of the enrichment process. A high platelet count compromises RBC separation. We therefore recommend reducing and standardizing the platelet count at the beginning of the process.
To support this recommendation, we performed an additional study to evaluate the benefits of reducing platelet counts before RBC separation, comparing:
i. MNC enrichment efficiency after platelet removal, followed by RBC separation
Intermediate bag
Final bag
ii. MNC enrichment efficiency directly after RBC separation on unit with high platelet concentration
Intermediate bag
Final bag
Upstream platelet depletion contributes to decreased RBC contamination during density gradient separation. This finding held true particularly when the initial product contained a high number of platelets.
Additional considerations:
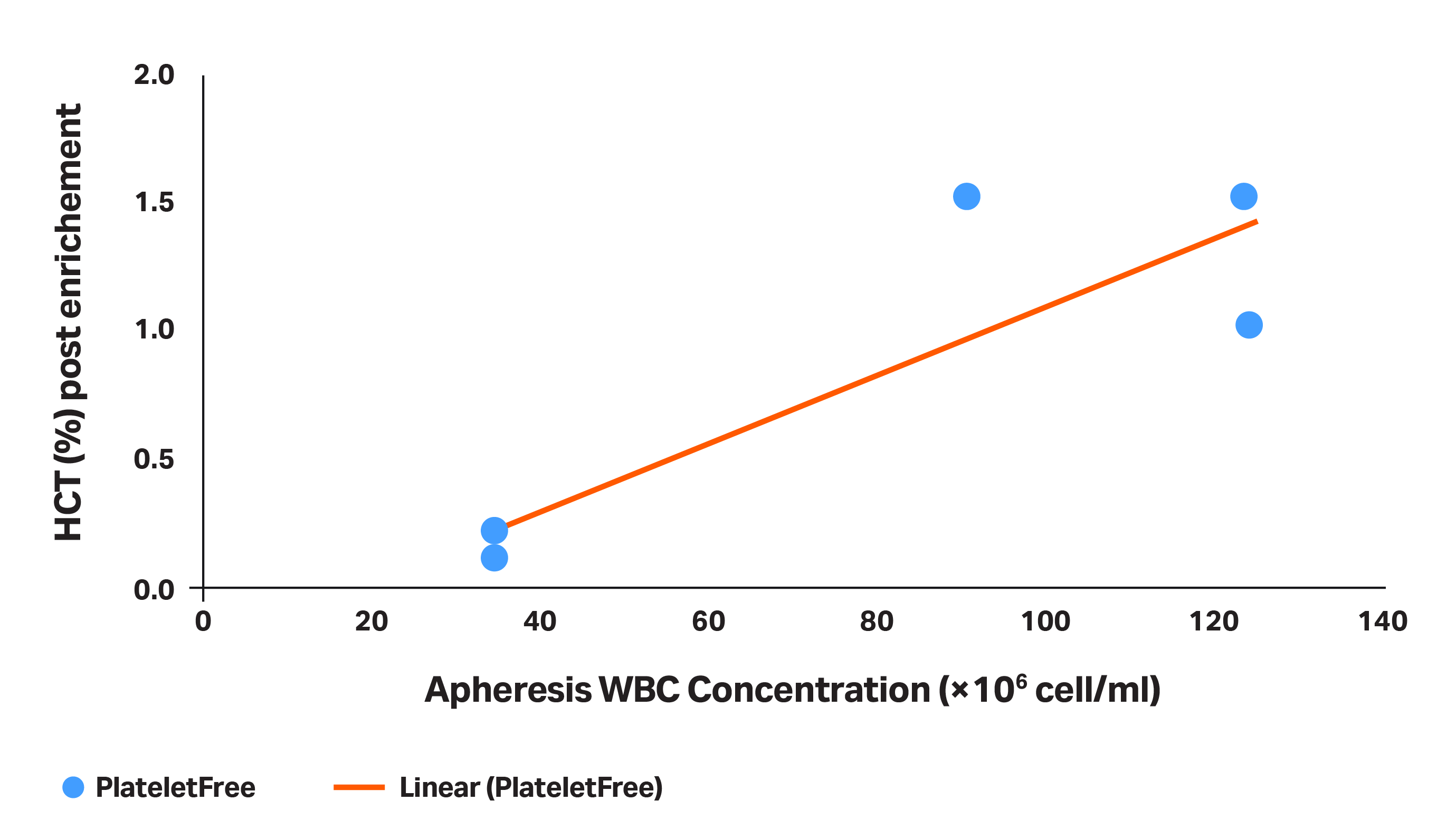
- Apheresis WBC concentration can impact efficacy of RBC separation. In addition to platelet count, WBC concentration in the initial apheresis product can impact the RBC separation success. As WBC concentration increases, so does risk of RBC contamination. For apheresis units with an initial WBC concentration higher than 80 to 100 × 106 cells/mL, you are unlikely to get the hematocrit of MNC-enriched product to be significantly lower than 1% (Fig 4).
Fig 4. The HCT (%) obtained in the product after the MNC enrichment (platelet depletion + RBC separation), as a function of WBC concentration.
- Collection centers can provide apheresis products with different characteristics. From our direct experience, apheresis providers have different sized pools of donors and collect products at varying frequencies. In general, the higher the frequency of collections, the higher the number of activated platelets in the donor material.4
Note: Some customers have reported issues related to Ficoll™ separation when switching from one collection center to another.
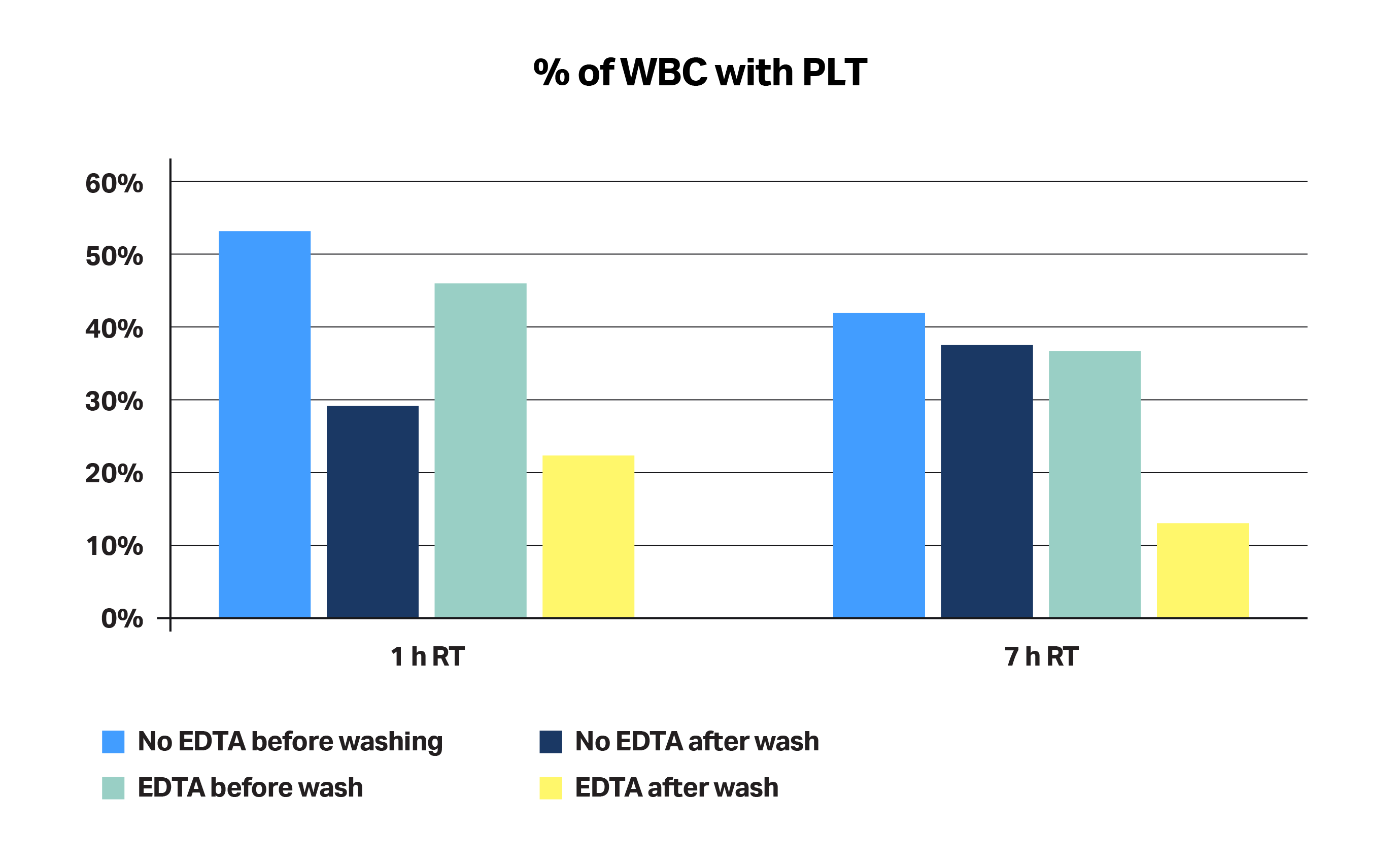
The type of buffer used can minimize risk of cell aggregate formation. Using a buffer containing a calcium chelator, such as EDTA, during apheresis product transport and washes minimizes the percentage of platelets attached to WBCs, thus decreasing the risk of cell aggregates having a negative impact on RBC separation (Fig 5). Apheresis products were collected from healthy donors and shipped at RT in less than 24 h. Analysis was performed by flow cytometry, looking at activation markers for platelets (P-Selectin/CD62-P). We found that, without EDTA (a calcium chelator), we lose the capability to wash away platelets over time.
Fig 5. The percentage of WBCs attached to platelets. Analysis was performed by flow cytometry, looking at platelet activation markers (P-Selectin/CD62P).
Additional recommendations to standardize MNC enrichment
Consider the following strategies to further reduce the variability of initial apheresis products:
- Dilute apheresis products in solutions supplemented with 1 mM EDTA (or other calcium chelator) just after collection to avoid platelet activation and clumping. The solution must be based on phosphate-buffered saline (PBS, without Ca++ and Mg++) or standard saline. Albumin or serum can also be supplemented to further aid in robustness and cell protection.
- Process material up to a maximum density of approximately 100 × 106 cells/mL to minimize cell aggregations. Ship and store products at refrigerated temperatures throughout the enrichment process.
Table 1. Details of the non-standardized (A) versus standardized (B) processes
|
Collection |
Shipment |
PLT depletion |
RBC depletion |
|
|
Non-standardized process (A) |
• Non-systematically use same collection device • Frequency of collection ~ every 6 to 8 weeks • No control on cell density post processing |
• Non-temperature controlled • Use of basic buffer for cell dilution/washes |
No | YES – First (and last) steps |
|
Standardized process (B) |
• Use same collection device • Frequency of collection ~ every 10 to 12 weeks • Max 100 × 106/mL density post-processing |
• Temperature controlled at 4°C • Use of buffer containing calcium chelator (EDTA) |
YES – First step, g-force/sedimentation optimized for PLT depletion | YES – Second step |
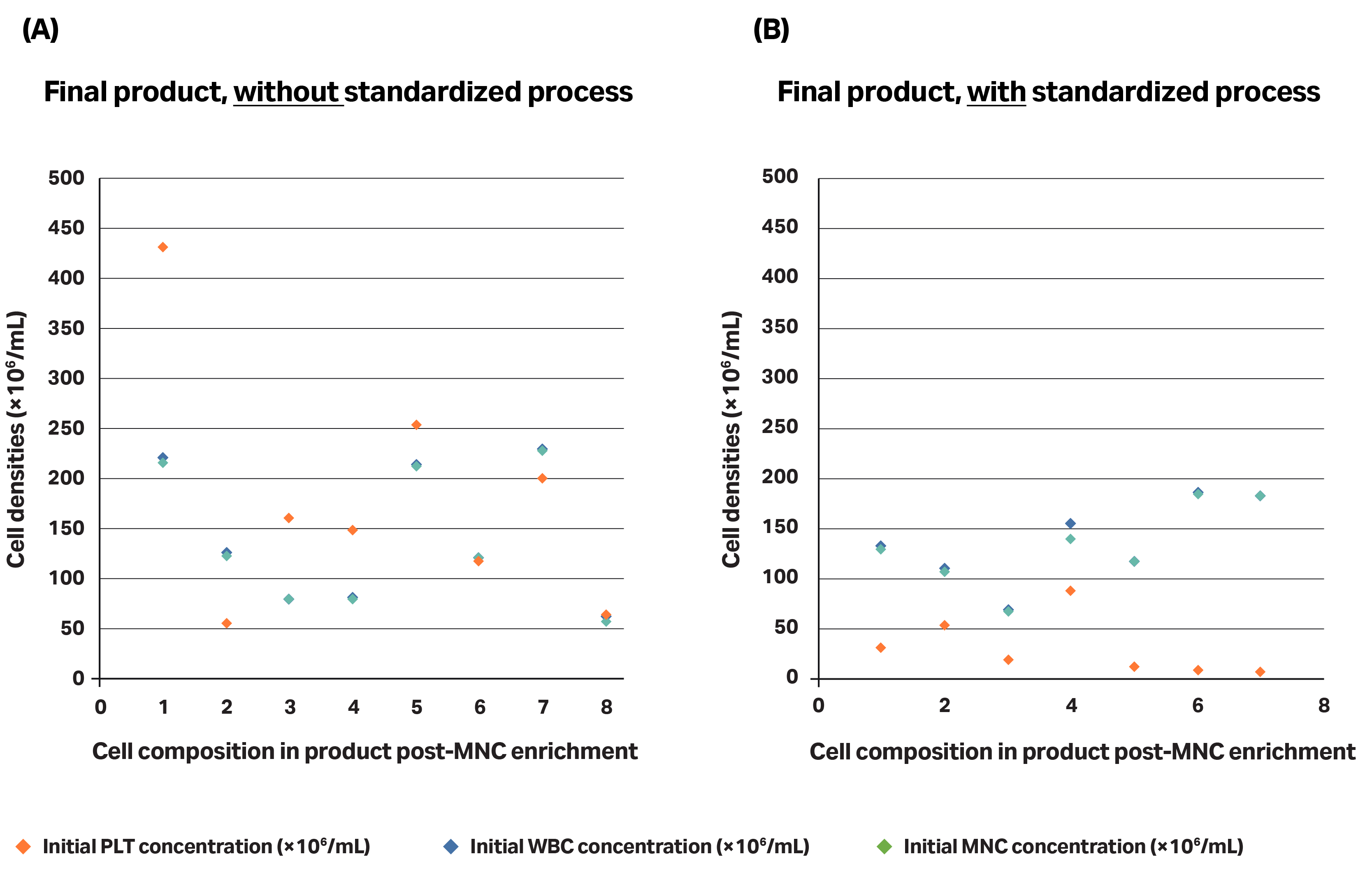
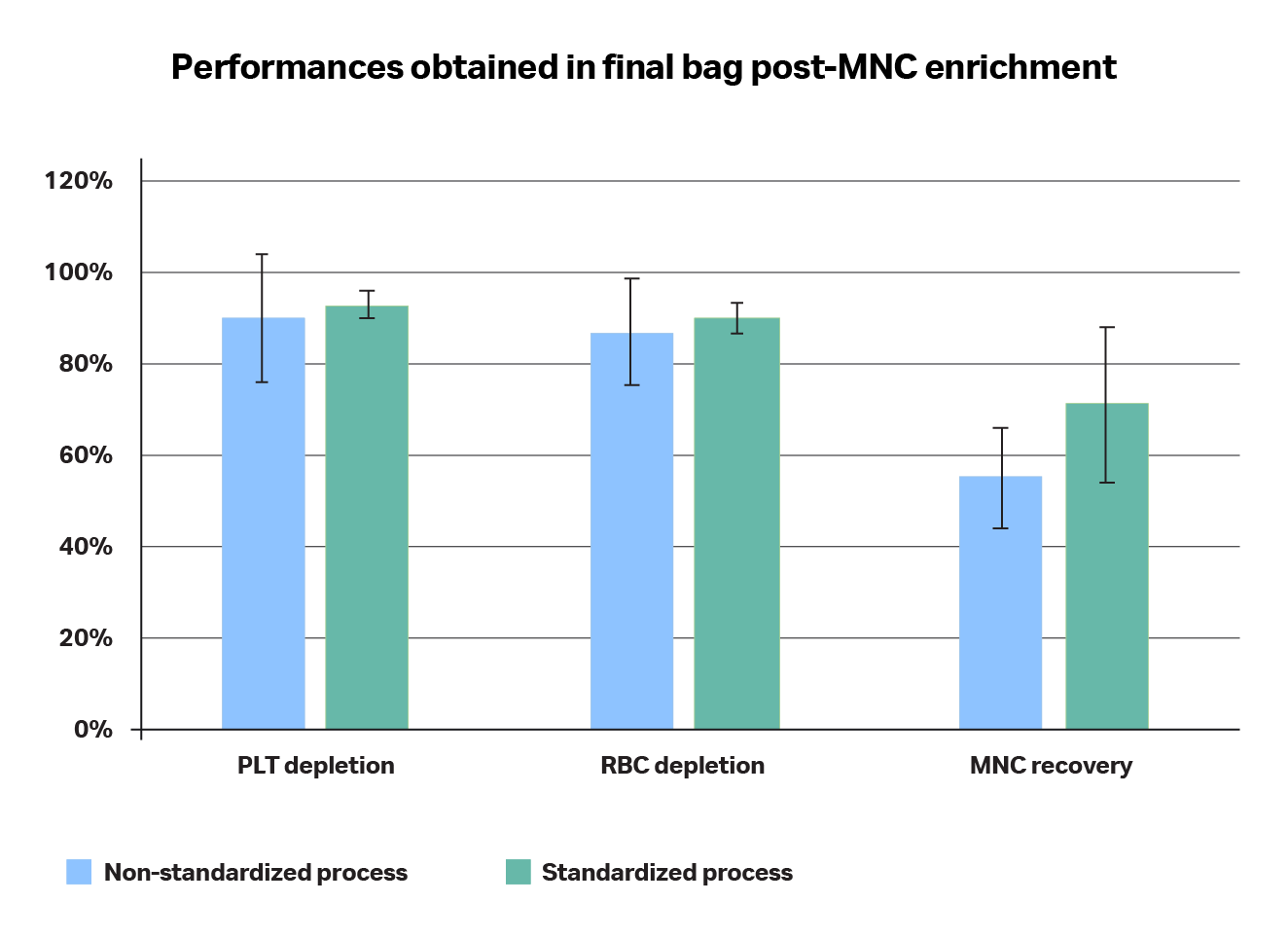
Fig 6. Comparison of the product obtained (in terms of cell content repartition) without (A) or with (B) a standardized process. (A) The non-standardized process did not follow recommended shipping conditions and is directly doing an RBC separation without upstream platelet depletion, whereas (B) the standardized process includes: the recommended collection and shipping conditions of the apheresis product and platelet depletion followed by RBC separation.
Fig 7. Comparison of performances in terms of RBC and PLT depletions and MNC recoveries when using a non-standardized (A) versus standardized (B) processes. The standard deviation in the non-standardized process is significantly higher than when using a standardized process and performances are systematically better when using a standardized process.
Automation of the MNC enrichment step/workflow
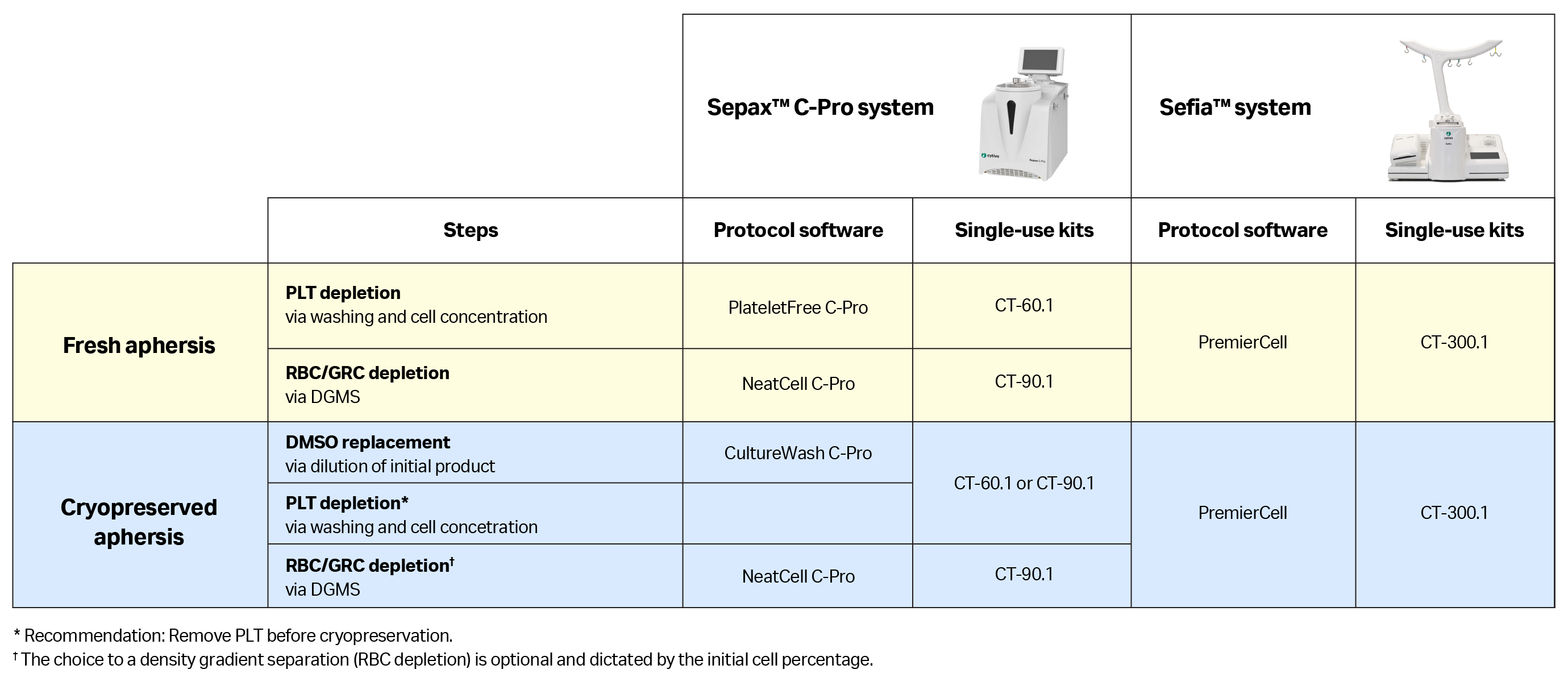
Once a standard protocol is applied for the MNC enrichment step, it is important to control for potential operator errors and contamination risks related to manual, open processing. Automated and functionally-closed processes have been developed to address this challenge. Several automation solutions can be applied, depending on whether the initial product is fresh or in a cryopreserved apheresis unit (Table 2).
- With fresh apheresis products, we recommend starting by removing platelets with a concentration step, and then removing RBCs and GRCs via density gradient medium separation. Both steps can be automated and closed using the Sepax™ C-Pro cell processing system, performing two consecutive procedures using the PlateletFree C-Pro and then NeatCell C-Pro applications with their dedicated single-use kits. If there is a need to perform both steps in a single procedure, it is possible to use the PremierCell protocol software on the Sefia™ system with its dedicated single-use kit.
- With cryopreserved apheresis units, an additional dilution step is needed to replace the cryoprotectant solution contained inside the cells and avoid its toxic effects at room temperature. Once the dilution is complete, platelet, RBC, and GRC removal can be performed. Note, platelet depletion is frequently done before cryopreserving the unit, and the RBC count might be small enough to not require a specific separation with density gradient medium
(Table 2).
Table 2. Summarizing all solutions, including device, disposable kit, and protocol software, for automated MNC enrichment from fresh or cryopreserved products
With the panel of solutions outlined in Table 2, we can standardize and automate the MNC enrichment process, despite the patient-to-patient cell composition variability in starting apheresis material.
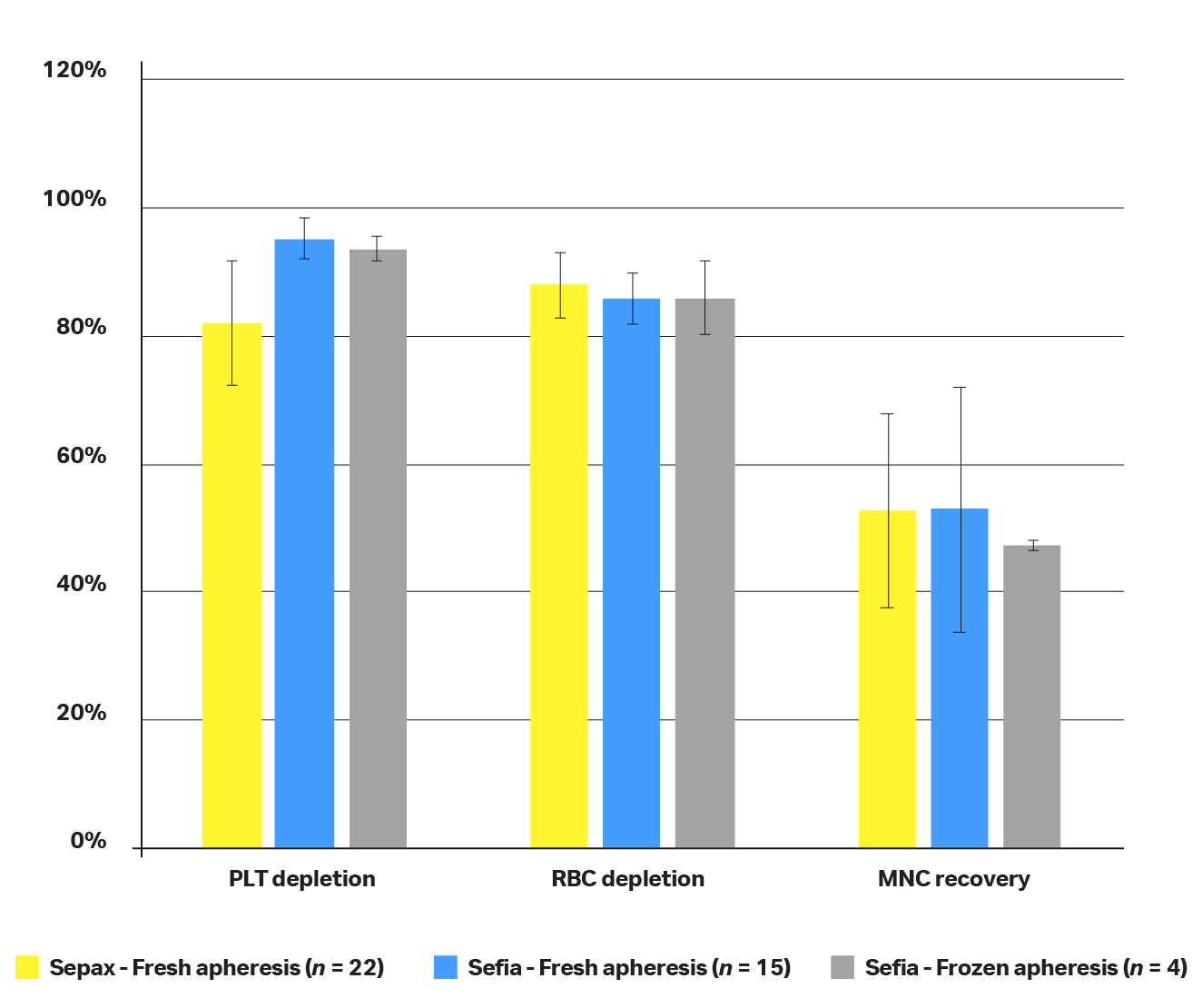
Fig 8. Performance comparison between the Sepax™ (yellow) and Sefia™ (blue) instruments using fresh or frozen input product. Leukapheresis product from different donors has been used to generate data obtained on Sefia™ and Sepax™ instruments: Leukapheresis product from n = 22 different donors was used to generate performances on the Sepax™ instrument, leukapheresis product from n = 15 different donors was used to generate performances on the Sefia™ instrument, and n = 4 different donors were used to generate performances on the Sefia™ instrument with frozen input leukapheresis product.
F-test and t-test have been performed to:
- Verify if variances were equals between Sepax™ and Sefia™ data sets (when starting with fresh and frozen apheresis product)
- Verify if performances obtained for RBC/PLT depletion and for MNC recovery were comparable between Sefia™ and Sepax™ instruments when starting with fresh apheresis product (Table 3)
- Verify if performances obtained for RBC/PLT depletion and for MNC recovery were comparable on the Sefia™ instrument when starting with fresh versus frozen apheresis product (Table 4)
The F-test revealed that variances were not equal between Sefia™ and Sepax™ instruments for RBC/PLT depletion or MNC recovery (when starting with fresh or frozen apheresis product).
Based on this finding, a first t-test assuming unequal variances was done comparing the performances of the Sepax and Sefia instruments when starting with fresh leukapheresis. Results (Table 3) revealed the following:
- When starting with fresh apheresis, PLT depletion on Sepax™ and Sefia™ instruments is not comparable — results obtained when using the Sefia™ instrument showed more than 10% PLT depletion compared to the Sepax™ instrument
- RBC depletion on Sepax™ and Sefia™ instruments is comparable
- MNC recovery on Sepax™ and Sefia™ instruments is comparable
Null hypothesis: Performances are comparable
Table 3. Statistical tests assuming unequal variances between Sepax™ and Sefia™ instruments when starting with fresh apheresis
|
t Stat |
t Critical two-tail |
Result |
Conclusion |
||
|
PLT depletion |
-4.742822367 | 2.144786688 | t Stat < - t Critical | Reject null hypothesis → Means are not comparable | |
|
RBC depletion |
1.148178789 | 2.055529439 | t Stat > - t Critical | Null hypothesis correct → Means are comparable | |
|
MNC recovery |
-0.038254698 | 2.119905299 | t Stat > - t Critical | Null hypothesis correct → Means are comparable |
A second t-test assuming unequal variances was performed to compare the Sefia™ instrument’s performances with frozen and fresh apheresis. Results (Table 4) revealed the following:
- PLT and RBC depletion performances are comparable when starting either with fresh or frozen apheresis
- MNC recovery performances are comparable when starting either with fresh or frozen apheresis
Null hypothesis: Performances are comparable
Table 4. Statistical tests assuming unequal variances between frozen and fresh apheresis on the Sefia™ instrument
|
t Stat |
t Critical two-tail |
Result |
Conclusion |
|
|
PLT depletion |
1.34217165 | 2.3060041 | t Stat > - t Critical | Null hypothesis correct → Means are comparable |
|
RBC depletion |
-0.0248962 | 2.77644510 | t Stat > - t Critical | Null hypothesis correct → Means are comparable |
|
MNC recovery |
1.02312526 | 2.22813885 | t Stat > - t Critical | Null hypothesis correct → Means are comparable |
Conclusions
The processing of source material for manufacturing cell therapies is a crucial step that influences the quality and efficacy of resulting treatments. Apheresis products used as input for CAR-T cell therapies have extremely high variability affected by many parameters including: the collection device used, the protocol used by the apheresis device, the operator who performed the collection, the temperature at which apheresis products are stored and shipped, and, most importantly, unique patient characteristics. The standardization of all parameters that are under our control is therefore of utmost importance
To minimize the different sources of variability that could impact input product, we have provided a list of recommendations to help standardize the processing of apheresis products from start to finish, based on published studies and the research presented in this report.
Our list of recommendations includes: using the same apheresis collection device from one procedure to the other, shipping apheresis products at 4°C in order to preserve cell viability, and standardizing the process of MNC enrichment.
The success of the MNC enrichment step is critical for the efficiency of the CAR-T cell therapy steps, especially T-cell isolation. We therefore recommend systematic enrichment of apheresis product at the beginning of the process by first removing platelets and then RBCs using a density gradient medium.
To minimize variability between procedures and operators, avoid contamination, and guarantee traceability, we strongly recommended the use of a closed and automated instrument for all cell therapy steps where possible, including MNC enrichment. Closed, automated processing will significantly reduce manufacturing time and ensure the traceability that is essential in the production of these sensitive, critical therapies.
References
- Watson Douglas, Parrington J, Dowsing C, et al. Initial Comparisons of Three Apheresis Platforms for Supporting the Collection of CD3+ Cells for CAR-T Production. Cytotherapy. 2016. 18. S11. 10.1016/j.jcyt.2016.03.028.
- Clarke D, Taylor B, Lawrence N. A Comprehensive Analysis of Fresh Apheresis Collections: Conclusions and Best Practices (White Paper). HemaCare Corporation. WP-APH-BEST-PRAC-V1.0. Accessed 2021 Jun 23.
- López‐Pereira, P, Sola Aparicio, E, Vicuña Andrés, I, et al. Retrospective comparison between COBE SPECTRA and SPECTRA OPTIA apheresis systems for hematopoietic progenitor cells collection for autologous and allogeneic transplantation in a single center. J Clin Apher. 2020; 35: 453– 459.
- AABB Standards for Blood Banks and Transfusion Services, 32th Edition. http://www.aabb.org/sa/standards/Pages/standards-programs.aspx. Effective July 1, 2020. Accessed October 29, 2020.
- Mathur G, Mott SL, Collins L, Nelson GA, Knudson CM, Schlueter AJ. Factors influencing platelet clumping during peripheral blood hematopoietic stem cell collection. Transfusion. 2017;57(5):1142-1151.
- Brosig A, Hähnel V, Orsó E, Wolff D, Holler E, Ahrens N. Technical comparison of four different extracorporeal photopheresis systems. Transfusion. 2016 Oct;56(10):2510-2519. doi: 10.1111/trf.13728. Epub 2016 Jul 26. PMID: 27456672.