Introduction
Cell therapy — defined as the transfer of autologous or allogenic material into a patient for medicinal benefit — is a promising and rapidly advancing field with the potential to transform multiple therapeutic areas such as regenerative medicine, immunotherapy, and cancer therapy.
Compared to traditional pharmaceuticals and therapeutics that are produced on mass scale in identical formats, autologous cellular therapies represent patient-specific products that are based on biological materials, which are inherently variable and unstable. Currently, most cell therapies are in the early stages of development. However, as more products enter the commercial landscape, there is an increasing need to consider the logistical challenges surrounding their delivery.
In this article, experts within the cell therapy space provide first-hand experience on how the combined complexity, variability, and rapid expansion of cell therapies creates challenges for the apheresis centers, clinical sites, and manufacturing facilities involved in their development. As well as outlining current challenges, they provide insight into some of the anticipated barriers to continued scale-up of cellular therapies and finally, go on to explore how the industry could develop solutions to meet future challenges in the field.
Site selection and accreditation
The success of any clinical trial is contingent on selecting appropriate and high-quality sites. So, before any clinical trial takes place, sites need to be carefully selected and audited in order to understand their capabilities and research experience. In a typical biopharmaceutical space, sponsors are looking for a well-trained, experienced clinical team that is able to deliver patients in a particular indication to fulfill clinical trial requirements.
While these factors are still relevant for a cell and gene therapy product, the additional requirements needed to ensure a successful trial add further complexity to process. Will Shingler, senior director of patient and cell management at Autolus, explains how site selection varies for cell therapies: “There's normally a very well-established process by which the GMP quality organization qualifies suppliers of raw material into that manufacturing process, involving procedures such as GMP assessments and audits. But what is the major difference between a cell therapy and a typical biopharmaceutical product? The fact that an autologous cell therapy product is a genetically engineered version of the patients’ own cells, and so essentially the clinical site becomes a supplier of raw materials into the GMP manufacturing process.”
He continued: “Therefore, to try and qualify the sites in the same way that you would qualify a supplier of an API [active pharmaceutical ingredient] into a manufacturing process doesn’t work.”
Apheresis for the collection of starting material (i.e., patient blood components) is one of the first critical steps in the cell therapy manufacturing process. Apheresis and the handling of patient cells requires specialist expertise and facilities. As such, “Clinical centers engaged in autologous cellular therapy tend to be centers that are experienced in blood and bone marrow transplant; so, they've got decades of experience in handling patients and their cells,” explains Will. “We're largely working with stem cell and bone marrow transplant centers and we're in the fortunate position that the vast majority of those centers are accredited by FACT-JACIE1. FACT-JACIE is an accreditation standard. It's a joint initiative of the American Society for Transplantation and Cellular Therapy (ASTCT), the European Blood and Marrow Transplantation (EBMT) and the International Society for Cell and Gene Therapy (ISCT), which have come together to define standards that show you a site is appropriately set up in terms of quality systems, training, and facilities to perform in the cell and tissue space.”
Although FACT-JACIE accredited centers are currently fulfilling the clinical roles needed for the collection and delivery of cell therapies, their capacity is limited. “I think for the clinical centers, certainly in terms of T cell-based therapies, apheresis capacity is going to be a significant challenge, just because the centers have, over the decades, established sufficient capabilities to manage their stem cell transplant programs. But, the new wave of ATMPs [advanced therapy medicinal products] coming through will significantly challenge the capacity of apheresis,” explains Will.
Increasing the number of locations with appropriate accreditation is an obvious solution; however, it's not straightforward. As Stuart Ings, processing facility director and designated individual at the Wolfson Cellular Therapy Unit of the University College London Hospital (UCLH) NHS Foundation Trust, explains: “There’s a lot of accreditations and education that clinical centers and their staff need to have to work in the cellular therapy space. From Human Tissue Authority (HTA) accreditation to procure the starting material, to Service Level Agreements (SLAs)2 required for other licensable activities which the site does not perform, such as some testing activities. In addition, all the sponsors I am aware of insist you are JACIE accredited, but gaining all these accreditations takes time. It can take years, as well as a significant financial investment.”
Additionally, it can be frustrating and time-consuming for clinical site staff to deal with consistent revisions of standard operating procedures (SOPs) to be inclusive of the increasing number of cell therapies. Equally, manufacturers face similar challenges, having to manage the site certification and the variations in clinical site operating models or organizational structures.
Leukapheresis and patient scheduling
Once a clinical site has been selected and the cell therapy trial has received approval from the relevant regulators, patients can start to be identified and recruited into the study. Integration of patient information and records into both the clinical sites’ and manufacturers’ systems is required before the study can begin. Then, for a Chimeric Antigen Receptor T (CAR T) therapy, the starting material is collected via leukapheresis by specialist nurses and doctors.
While this may sound straightforward, patient scheduling can be logistically challenging. A high degree of coordination between multiple touch points (clinical sites, manufacturers, cryopreservation facilities, couriers, etc.), often involving large amounts of manual administration, is required. Will highlights that “The biggest piece from a logistics standpoint is making sure that we understand when that patient will be ready for apheresis. This will need to be aligned with the manufacturing side and careful coordination between the clinic and the manufacturers is essential. Basically, we need to ask: one – when will the patient be ready? And two – when do we have a manufacturing slot?”
Once this information is known, the appropriate teams — from apheresis and laboratory staff to cryopreservation personnel and couriers — can be coordinated. However, as Will explains, “patient scheduling is always a challenge. For example, particularly in a clinical trial setting, you can have unexpected delays to the process. A patient’s liver enzyme results could be out of specification or the patient might be too weak to travel, so you can't collect them on that day and you need to reschedule. However, the established apheresis centers are used to being very agile because their history is around the collection of stem cells. Nevertheless, any delays here create downstream delays to the supply chain and manufacturing process. All the couriers, laboratory staff, and equipment will need to be re-booked or re-scheduled.”
At his clinical site, Stuart outlines that patient scheduling is often done at the last minute: “We work very closely with the apheresis teams here to timetable appointments as we have to be exceptionally careful with our timings since they’re frequently very urgent referrals.”
The time constraints imposed by the perishability of cell therapy starting material places further complexity on scheduling. With cell therapies, there is a fixed ‘vein-to-vein’ time, meaning that success is contingent on the delivery of starting materials and final therapeutic in a set timeframe. Additionally, starting materials and final products must be strictly temperature controlled. Consequently, tight scheduling between numerous people and departments is a necessary but challenging task.
Transport logistics
Autologous cellular therapies are complex and personalized, creating numerous challenges in the standardization and logistics of their manufacturing and supply chain. Adding to the complexity is the perishability of both starting materials and final products that demand detailed transport processes and requirements. While transport logistics may change depending on the nuances of the specific therapy, such as whether the transported material is fresh or frozen, procedures across cellular therapies can be generalized.
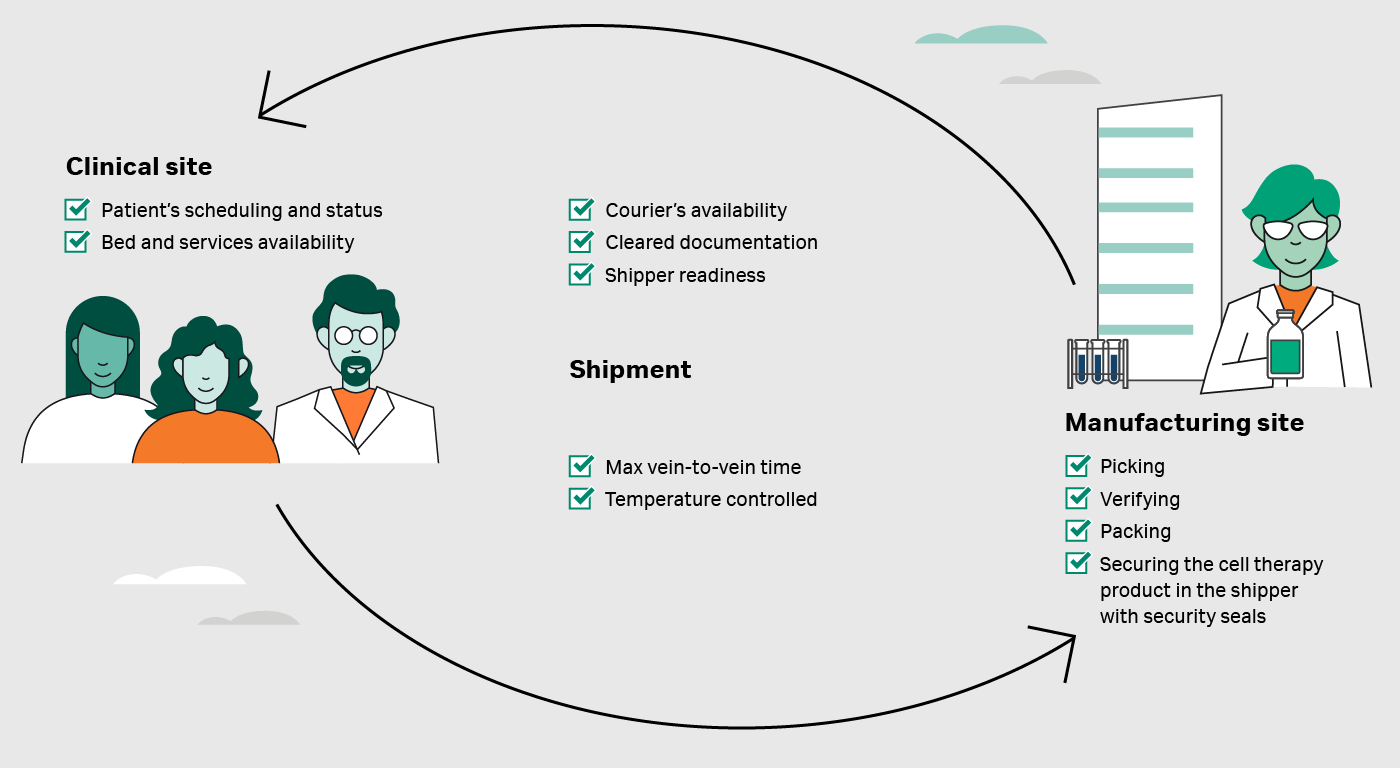
Chris Fong, senior director of distribution and logistics at Autolus, provides a brief overview of the process required to ensure the transport of cellular therapy starting material to the manufacturing site: “At the point that the patient has been scheduled for apheresis, we will start our booking with a courier,” explains Chris. “First, we need to arrange the delivery of a preconditioned temperature shipping box to that clinical site. This is usually delivered in the morning, so it will be waiting while the patient undergoes apheresis.”
He continues, “When the collection of cells is done, they'll be transferred to the preconditioned dry shipper and then it's tendered over to the courier. The clinical site signs off, the driver signs off, the packaged shipper is handed over, and that driver then takes it on its journey.”
Once the starting material reaches the manufacturing site, cells are expanded and genetically modified to produce a final therapeutic product. Chris continues “When we start to get close to the point at which the therapeutic is ready to be shipped back to the patient, we conduct QC [quality control] tests to verify the drug product meets quality standards and is suitable for treatment. This helps us estimate a planned date for manufacturing completion. If it’s all on track, there will be some initial coordination by our manufacturing or scheduling team to book a temporary slot for cryopreserved shipping. As we get closer to having a manufactured drug product, we can confirm the booking and have the courier deliver a shipper.”
Shippers for cryopreserved materials need to be temperature preconditioned, typically with liquid nitrogen. In other words, the internal temperature needs to reach the appropriate level (e.g., -150˚C). Thus, the shippers have a limited lifespan and need to be carefully coordinated and scheduled with manufacturing completion, logistics duration, and clinical site receipt. It can be difficult for manufacturers to precisely schedule shipper delivery in advance since there may be delays to manufacturing or QC.
Once the shipper has been delivered and the QC results indicate the cellular therapy has met the standards required for patient treatment, the transport leg to the clinical site can commence. At the manufacturing site, staff will “go through the process as far as picking, verifying, packing, and securing the material in the shipper with security seals. Shipping documents are completed with a signature at the point it is tendered to the driver,” outlines Chris.
He continues “Throughout the transit to the clinical sites, there are specific milestones to oversee and possibly intervene to ensure that the plan is carried out as expected. For example, if it's a flight, was it tendered over to the airline as expected? Did the shipment make it onto the flight? Did it arrive at the airport on time? Did it complete clearances? Is it on the final leg to the clinical site?”
Fig 1. Example of practical challenges and points to be verified through an autologous cell therapy journey.
The cellular therapy should arrive at the clinical site with all required documentation, allowing staff to verify receipt and sign-off with the driver. The clinical team have a series of verification checks to carry out prior to transferring the starting material from the shipper into appropriate storage. Finally, when appropriate, the patient will be preconditioned for their product and, ultimately, delivered the therapy.
While the transport flow of gene therapy manufacture may have defined steps, the perishability of cellular materials means the process is particularly sensitive to delay. These delays become increasingly likely with more distance and/or the necessity to transit across multiple borders. Chris’ personal experience at Autolus highlights the complexity added by international tender: “Our [clinical trial] study spans different countries. We have to deal with factors such as export, air flights, exchanges, and tendering between different agents, customs, and then final delivery. With all these events and processes, there is potential for delays. And with COVID, it's been even more challenging.”
Delays are not just confined to transit. “We can run into situations, for example, where there's some delays with manufacturing. The QC process may indicate further evaluation and tests are required. We understand our patient is waiting for therapy, and delays must be mitigated as much as possible,” Chris explained.
He goes on to discuss how delays can have logistical consequences: “When it comes to the LN2 [liquid nitrogen] shipper, it's a perishable item. At the point the couriers condition it, the clock starts. We have about 10 days of duration. That’s 10 days that it'll hold that temperature. However, there’s often variation between when we expected something to be ready and reality. There are also other factors like weekends, receipt hours/dates, or pack out dates that can shift. So, we can end up in a situation where we have to return that shipper and get another one, or we have to use another shipper intended for a different shipment.”
Delays not only complicate logistics but, ultimately, they may have potentially catastrophic impacts on vulnerable patients. Will explains, “With an autologous cell journey you've got a sick patient waiting for treatment and so you want to minimize the cell journey from the point of patient identification or cell acquisition through to reinfusion of the product. And that obviously means there's some significant urgency in your logistics, which doesn't leave a lot of room for error. Any kind of slack that you build into the system is potentially viewed as detrimental. Essentially, you are always racing against the clock to get the therapeutic to the patient.”
Integration and coordination between departments
It is important to consider the diversity of specialists and departments that need to be coordinated throughout the manufacturing cycle. For instance, collection of starting material relies on more than just the apheresis team. “The lab will need to do peripheral blood and CD3 (a marker of T-cells) counts to make sure that we're going to be processing enough blood to collect sufficient cells,” explains Stuart. After collection of cells and packaging the starting material, courier teams will need to be available and aligned with the manufacturing site.
This scenario is also mirrored when it comes to treating patients, as Will explains: “With CAR T cell therapies, patients can experience Cytokine Release Syndrome (CRS), which can make them unwell. If it’s particularly severe, they can develop Immune Cell Associated Neurotoxicites (ICANs). Because of this, the treating center needs to reserve an ICU bed, just in case. Then, you have to consider all the other required departments — from the pharmacy to neurology and hematology. All these departments will need to have established relationships to ensure the treatment is executed safely and effectively.”
The complications in logistics vary between cellular therapy products. Helen Delahaye, managing director and founder at Deltohn Limited, has had extensive experience working with meniscal tear cellular therapies. She outlines that “while CAR T cell therapies rely on a specialized nursing team to deliver the product to the patient, regenerative medicine treatments may require specialist surgeons. For instance, an orthopedic surgeon is required to give a meniscal tear cellular therapy transplant. Because there are a limited number of orthopedic surgeons with the right skillset, scheduling becomes even more difficult.”
Cryopreservation
Cryopreservation, the preservation of biological samples at very low temperatures, is often an integral part of cellular therapy production. These extremely low temperatures (≤120°C) extend the shelf-life of cells or tissues by inducing a reversible state of suspended cellular metabolism. Cryopreservation can be used to preserve the starting cellular material before beginning large-scale manufacturing and, in most cases, is applied to the final therapeutic product prior to shipment to the clinical site.
Cryopreservation provides undeniable benefit to cellular therapy manufacture and supply chain. However, there are some limitations and, as Will explains, these need to be thoroughly examined when deciding whether to cryopreserve starting materials: “The obvious advantage of dealing with frozen material is that once it's collected and frozen, it's almost indefinitely stable. You can queue it into manufacturing where you might have a few lined up and they can go into the first available manufacturing slot. The disadvantage of cryopreservation of starting materials is that you will inevitably get some cell loss through the freeze-thaw process. And, depending on the success of the collection and the ‘vulnerability’ of the patient and their immune system, you might find downstream manufacturing issues.”
He continues, “At Autolus, we currently manufacture directly from fresh cells, which has the advantage of not putting the cells through that freeze-thaw trauma, but has the disadvantage of directly linking a collection on a particular day to a manufacturing start on a particular day. When you have changes in the apheresis schedule there is a direct impact on the manufacturing schedule.”
Cryopreservation of starting materials also places additional burden on clinical sites, which need requisite equipment and processes, appropriate handling and knowledge, and trained staff and expertise. Will notes that “the infrastructure around cryopreservation and liquid nitrogen is fairly large. You need a storage tank to refill your freezers and the freezers themselves need to be fairly large.”
Consequently, clinical sites need to have the space to house such facilities, a factor that may prevent some clinical sites from ever having the capacity to cryopreserve. Moreover, even if space permits cryopreservation facilities, the financial burden represents a major barrier to implementation.
Stuart adds “Being a routine stem cell lab, we are used to cryopreservation of patient materials. However, we are working with cell therapy sponsors who require cryopreservation of starting materials, which can present some additional challenges. Firstly, one sponsor insists that we use a controlled rate freezer, which we do not use for our standard products. For stem cells, we use overnight freezing in a -80°C freezer, which is relatively easy and scalable. In contrast, to freeze five or six patients’ materials in controlled rate freezers, you’d need five or six controlled rate freezers. We don’t have the capacity to do this.”
John Davidge, clinical trials manager of Newcastle Advanced Therapies at Newcastle upon Tyne Hospital NHS Foundation Trust, explains how even at larger clinical sites, they’re running out of capacity: “We’re quite lucky at our facility because we have quite extensive infrastructure, including four liquid nitrogen tanks. In total, we’ve got capacity for over 2000 units to be stored in liquid nitrogen. But we’re reaching the limit of storage now, which could become a problem.”
Will adds an important consideration: “Wrongly handled, nitrogen can kill people and has done. Ensuring people are trained correctly and the protocols are in place to keep people safe is therefore of critical importance.”
Stuart summarizes, “To cryopreserve starting materials and store cryopreserved final products, you have to have the space, the infrastructure, and the staff. Not only do you have to buy freezers and nitrogen tanks, but you have to have regular nitrogen deliveries and all the safety measures, such as oxygen monitors and, potentially, also carbon dioxide monitors.”In addition to specialized equipment, staff require appropriate training to work with cryopreserved materials. Collectively, this translates to significant investment, as Stuart highlights from personal experience: “We brought a vapor phase nitrogen tank because a sponsor needed us to guarantee a temperature of ≤155°C. We had to buy a tank and also a back-up tank in case the original breaks down. We have to get them validated and all of this costs tens of thousands of pounds — a significant amount of money to the NHS trust.”
Chris notes that, as cellular therapies scale-up, there may be challenges associated with expanding to more rural or isolated areas. “Most clinical trials are being and going to be conducted in the larger markets — the ones that are more mature. But once they go to the next tier, it's going to be challenging for them to access more rural or remote regions. As far as capabilities and certain networks, if you're in a more remote area, it's less likely there’s access to infrastructure such as LN2 charging.”
He adds “I think when you start to access other regions that are not as experienced or haven’t handled LN2 shipments, which are very sensitive from a time and temperature standpoint, you're also going to have some risk. They're not necessarily going to have specific processes or services available for you.”
Maintaining chain of custody and chain of identity
There is a lot of documentation associated with the cell therapy manufacturing process — from temperature monitoring logs and sample and product transport tracking, to receipt of materials and laboratory reporting. Importantly, maintaining the chain of identity (COI) and chain of custody (COC) through proper documentation is crucial in autologous cell therapy manufacturing to ensure the patient receives the product manufactured from their own cells (COI) which have been maintained under the predefined appropriate conditions all the time (COC). This requires tracking of both the materials and its metadata (e.g., temperature) from receipt of the patient cells to manufacturing and finally to patient delivery.
Will explains how the legislation and regulation surrounding cellular therapies has caused confusion about who has the responsibility to maintain COI and COC: “There was a gap in the regulations governing patient-derived materials and that was filled by the tissue and cell directive in the European Union and the Human Tissue Act in the UK. It’s a piece of legislation that covers traceability, safety, consent, and procurement. The chain of identity and chain of custody have to be maintained by the facility who collected the cells. However, when you get to a manufactured ATMP product, a bit more of the onus falls upon the manufacturer. Because you're taking one product and you're transforming it into something else, we've ended up with a fuzzy grey area where the tissue and cell legislation stops — or maybe it doesn't — and the medicines for human use legislation takes over. Trying to define exactly which bit of which legislation applies to which bit of the cell journey is challenging.”
He adds, “But essentially in the manufactured ATMP space, it's up to the manufacturer to ensure that there is traceability and chain of custody. The sophistication of that depends on the manufacturer and their stage of development.”
Keeping detailed and accurate documentation is a challenging task for both clinical sites and manufacturers. Will explains that “in earlier stages of development, before you have a sophisticated cell orchestration platform to manage chain of identity and chain of custody, people are going to be using paper.”
Chris adds, “We’re still using paper [at Autolus], in the form of airway bills to keep chain of identity/chain of custody. We have been working with our couriers to make the transition towards electronic airway bills, but it’s not been as straightforward as you would expect. GPS is also used to for traceability and tracking. Additionally, at the minimum you're going to need a customs invoice and special handling requirements. Currently, that's all manually done. While they are submitted electronically, the paperwork still transits with the shipment. An online digital system would be preferable.”
There is a concerted effort in the industry to develop digital systems to manage documentation. Will explains, “There are lots of well-intentioned industry working groups that are looking at trying to make the life of our clinical sites easier. And, there are a number of digital cell orchestration platforms available. However, currently, clinical sites are working with multiple different sponsors, all potentially using different systems.”
Stuart elaborates on the complexity of having multiple digital systems at UCLH, “We're involved in about 20 trials and so have to handle numerous different sponsor systems. Consequently, in some ways, a paper-based system is easier. We're finding that we have to be trained on all these online systems and portals. They all have different logins, passwords, interfaces, and navigation. In practice, it’s extremely time-consuming to navigate so many different platforms. Moreover, our staff need to be trained on each software, which adds additional time investment to the process.”
While it is clear the future of documentation is digital, there are still some problems that remain with its implementation. Amisha Desai, lead pharmacist for Clinical Trials at University Hospitals Birmingham NHS Foundation Trust, explains, “Sometimes we’re managing deliveries in places where there isn’t a computer, WiFi connection is poor, or we don’t even have a telephone signal. We then have to remember to enter all the details retrospectively — using a different system for each different product — when we return to the office. It is not only time-consuming but opens up the process to human error.” Stuart echoed this challenge in his own facilities, noting that “our cryostorage facility is in a basement and my lab does not currently have WiFi.”
Amisha continued, “The connectivity issue is also a problem in areas other than delivery. Let’s say you’re opening a product at the bedside. It would be preferable to download the temperature data, for instance, there and then. But, without the proper additional IT infrastructure at clinical sites, it isn’t possible.”
Solutions and outlook
A transition toward allogenic therapies
Currently, all commercial and nearly all clinical trial stage cell therapies are autologous. However, allogenic cell therapies, which rely on a single source of cells to treat many patients, have numerous benefits and could significantly simplify logistics.
“With autologous therapies you have the burden of responsibility to get the cells back to the patient and therefore, maintaining the chain of identity is critical,” commented Helen. “Looking to the future, we are hoping to see more and more allogenic therapies. Not only do you remove the issues associated with maintaining chain of identity, but allogenic therapies offer an ‘off the shelf’ approach. For example, if someone comes in with a repairable meniscus, they don't have to start a two-week process of taking bone marrow, expanding cells, making a cell bandage, and sending it back to the patient for insertion. Instead, the clinician can just go into a freezer, as they would to get a titanium plate, for example, and put it into the knee.”
Universal digital platform for documentation
The creation and adoption of a universal digital platform for documentation and maintaining the COI and COC is the only clear solution to help clinical sites handle multiple cellular therapies. Stuart explains, “What would be lovely is if some of these sponsors and commercial manufacturers used the same or very similar systems. It would make the life of clinical sites so much easier.”
Although there are cell orchestration developers and platforms available, the industry needs a way to unify these diverse systems and establish some standardization. “There’s an ongoing initiative with various partners to create a universal portal for clinical sites,” explains Will. Each cell orchestration platform would dock into the back end of the portal via an Application Programming Interface (API). “But it's not yet clear it will gain the critical mass needed to be adopted by everyone. In other words, if enough manufacturers adopt the platform, it will force universal use.”
Chris adds, “We need some cooperation between all parties involved. In the UK, healthcare dynamics are more government initiated compared to places like the US. Therefore, areas like the UK might be in a better position to drive standardization — which may hopefully disseminate across the rest of the world.”
Investment in infrastructure and training
As cellular therapies scale up, it is apparent that appropriately accredited clinical sites will quickly become overwhelmed. “There would need to be significant investment in infrastructure, staff, and training,” explains Amisha. “Currently, we’re seeing the third wave of CAR-T sites in the UK. Sites are undergoing training and gaining accreditation to ultimately increase the footprint for CAR-T delivery — presumably to help with the myeloma product that's coming on board soon.”
Stuart adds, “To truly scale-up, we’ll need serious investment — building new labs and cryogenic storage for example, as well as training and employing new staff across multiple disciplines.”
Amisha continues, “Training is a really important aspect of expansion. I’ve been part of the ATTC3 and developing some of the educational materials that provide a resource for clinics. There is going to be a need for a huge amount of support for the upcoming third wave of NHS centers to be commissioned to deliver CAR T from first- and second-wave centers already experienced in this domain.”
Satellite sites for cryopreservation
As the infrastructure and staff needed to conduct cell therapy trials are highly specialized, there are currently a restricted number of clinical sites available. While significant investment in clinical site infrastructure and employment remains important, it is also possible to increase capacity by partnering different locations with different specialties.
Stuart describes sponsors that are setting up centralized labs for cryopreservation. “Some stem cell laboratories or biobanks have set themselves up to cryopreserve starting materials for the sponsor. Essentially, centers around the country can send their cells to these laboratories or biobanks, who would then cryopreserve and forward them on to the sponsor. I suspect that we’ll see more of this type of collaborative setup in the future.”
Will adds, “I think certainly there will be some sites that aspire to treat patients with cellular therapies that don't necessarily have the apheresis capacity internally. There's some thought that has been put into it in the industry and there are network apheresis centers already established. For example, both the American Red Cross and NHSBT [NHS Blood and Transplant] have plasma centers in the UK where they’re using apheresis machines to collect blood components for non-cellular transplants/therapies.”
He continues, “There’s definitely potential for that infrastructure to be taken advantage of for the collection of cells for the manufacture of ATMPs, to then support treatment of patients at centers that don’t have the specialist capabilities. For this to be a reality, we need to make sure that the patient can be managed accordingly, especially if they have an adverse reaction. But I think it’s definitely somewhere the industry is heading because there are advantages to this networked approach, such as the patient not needing to travel long distances and not having to be maintained as an inpatient for quite as long as they currently do. In turn, health services can make cost savings for reducing inpatient stays.”
Alternatives to liquid nitrogen
Over several years, liquid nitrogen (LN2)-free systems for controlled-rate freezing and transport of cryopreserved materials have been developed. These use electricity to power a Stirling Cycle engine as a cooling source to reduce temperatures to cryogenic values.
LN2-free systems can overcome some of the safety concerns associated with handling liquid nitrogen, as well as some of the related logistical issues. “I think the key advantage of LN2-free systems is access to clinical sites that don't have established cryostorage facilities. For example, with the VIA Capsule™ system from Cytiva, we can ship the final product to the clinical center, and they can receive it and plug it in. It essentially becomes a cryofreezer at the site. That's definitely got to be an advantage, particularly in the future when we move to sites that aren't set up in the same way as the established transplant centers,” explains Will.
Collaboration is key
The cell therapy landscape is highly dynamic and continues to change in light of emerging, diverse products. Stuart commented that “the use of CAR T cell therapies is going to go up and up and up. It's increased exponentially over the last few years. It's phenomenal, and it's only going to continue that way.”
However, the excitement surrounding cell therapies must also be met with a continuous search for solutions that allow these therapies to be delivered safely and effectively. It is only through collaborative global efforts that the potential of cell therapies to transform medicine across disease areas will be fully realized.
Want to learn more about cryopreservation methods? Click here.
Footnotes
1FACT: Foundation for the Accreditation of Cellular Therapy; JACIE: Joint Accreditation Committee
FACT-JACIE: International Standards for Hematopoietic Cellular Therapy (Product Collection, Processing and Administration)
2A service level agreement (SLA) is an agreement that sets out the roles and responsibilities of two parties. If two establishments are licensed by the HTA and one undertakes licensable activities on behalf of the other, an SLA is sufficient to document the working relationship between the two licensed establishments. In scenarios where one partner is licensed by the HTA and the other is not, a third-party agreement is required. It should be noted that some licensable activities are not permitted to be performed with a 3rd party agreement e.g., storage.
3The Advanced Therapy Treatment Centers (ATTC) is a network program operating within the NHS framework and coordinated by the Cell and Gene Therapy Catapult to address the unique and complex challenges of bringing pioneering advanced therapy medicinal products (ATMPs) to patients. For more information, visit: https://www.theattcnetwork.co.uk/