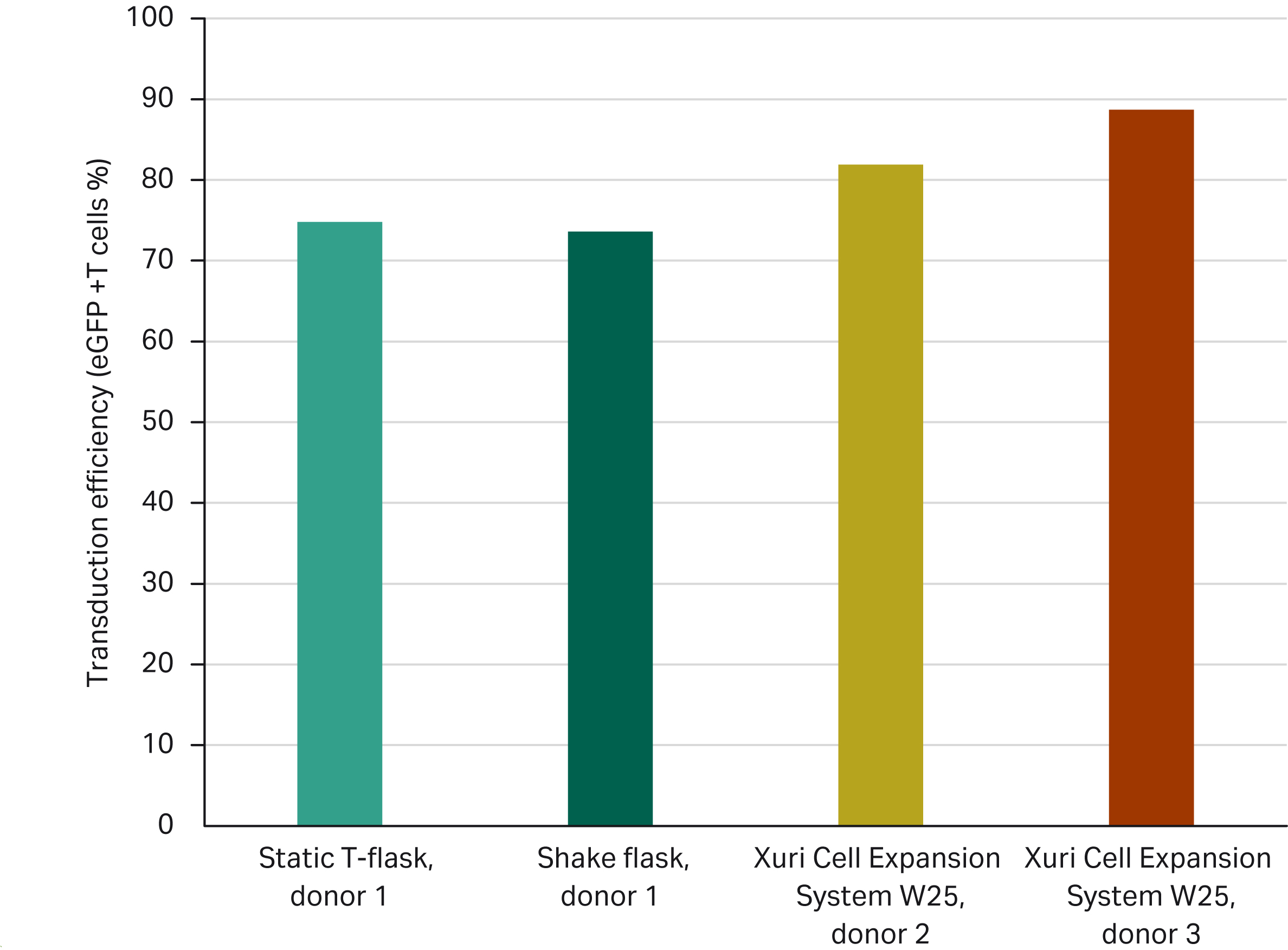Updated August 2023
Study overview: Automated spinoculation for gene transfer by lentiviral transduction
There is a growing need to close and automate manufacturing of chimeric antigen receptor (CAR) T cell therapies. CAR T workflows depend on ex vivo gene transfer by lentiviral transduction for therapeutic efficacy. To streamline this step while keeping flexibility during process development, we introduced stand-alone application software that uses existing Sepax C-Pro technology.
SpinOculation C-Pro application software automates lentiviral vector (LVV) transduction and maintains a functionally-closed system within a single-use disposable kit; importantly, no transduction enhancers are required. We present different use cases highlighting the flexibility of the SpinOculation C Pro software parameters for process optimization of LVV transduction efficiencies in combination with application-specific upstream processes and viral sources.
Introduction: The need for closed and automated lentiviral transduction steps in CAR T cell therapy workflows
Clinical and biopharmaceutical communities are undergoing an evolution in cancer treatment with the development and use of T cell immunotherapies. The approval of autologous CAR T cell therapies from Novartis (Kymriah™) and Gilead/Kite (Yescarta™) emphasizes the growing need for automated and closed solutions for cell and gene therapy manufacturing. Industrialization of these processes will require enhanced risk management, scalability, and reproducibility.
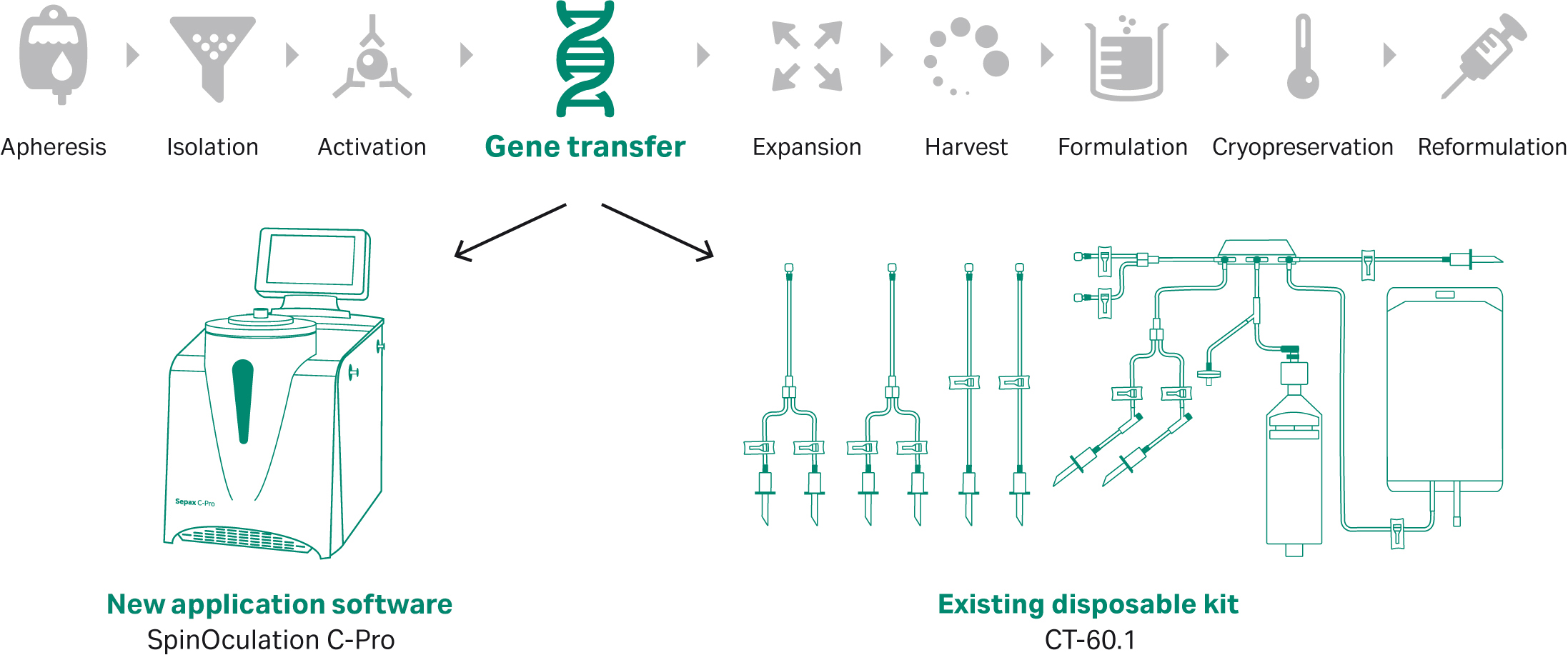
Although automated equipment is available for most workflow steps of CAR T cell therapy manufacturing, others, such as lentiviral transduction for gene transfer, need improved methods and technologies. SpinOculation C-Pro application software, used in combination with the Sepax C-Pro instrument and the CT-60.1 single-use kit, is a new stand-alone solution developed to streamline and automate lentiviral transduction while maintaining flexibility during process development (Fig 1).
The SpinOculation C-Pro application software allows users to adjust input cell volume to reach a specific cell density during the transduction step. Also, the application provides closed-system centrifugation to aid in gene transfer without the use of physicochemical transduction enhancers. The final output volume is adaptable to customer-specific needs.
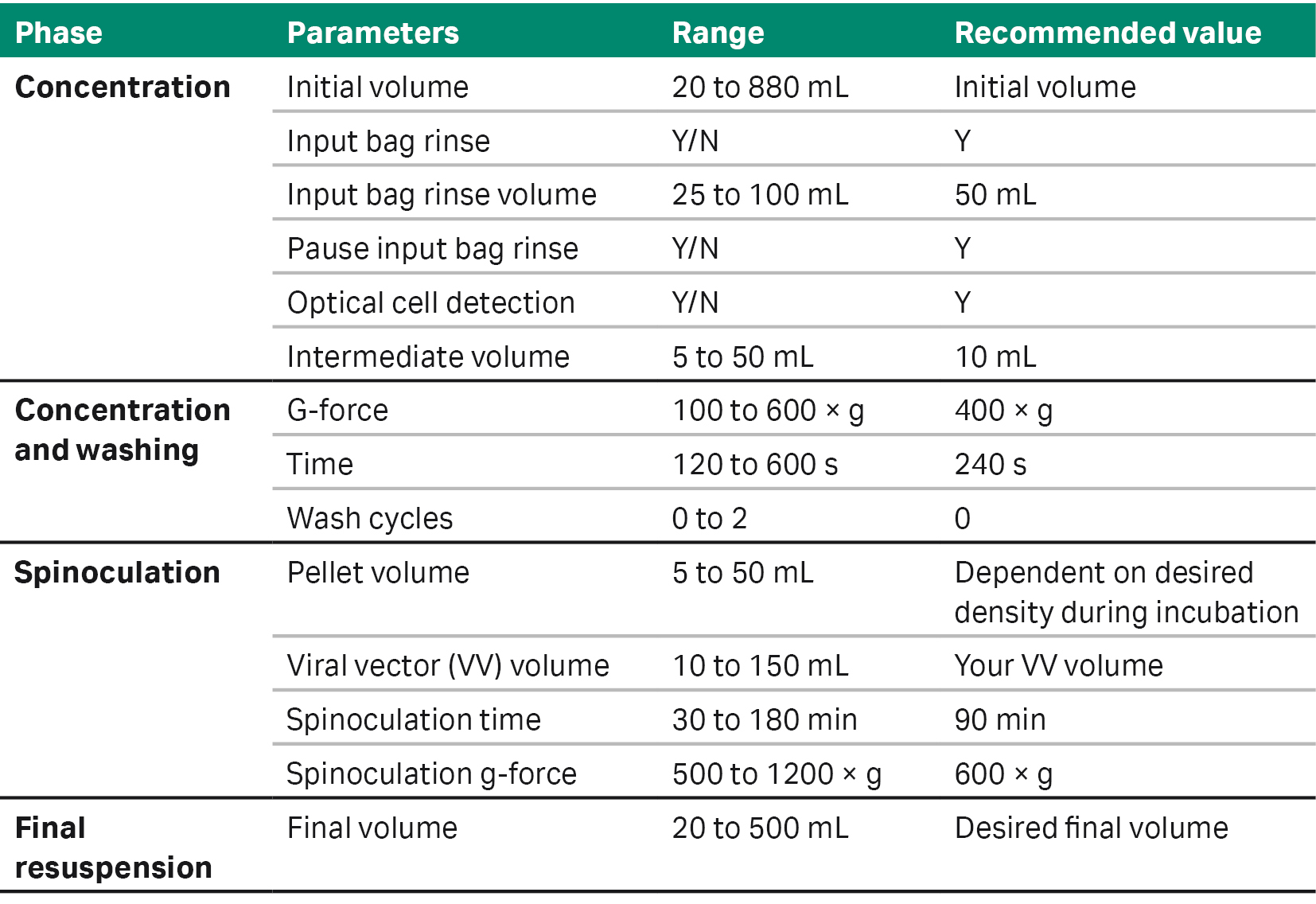
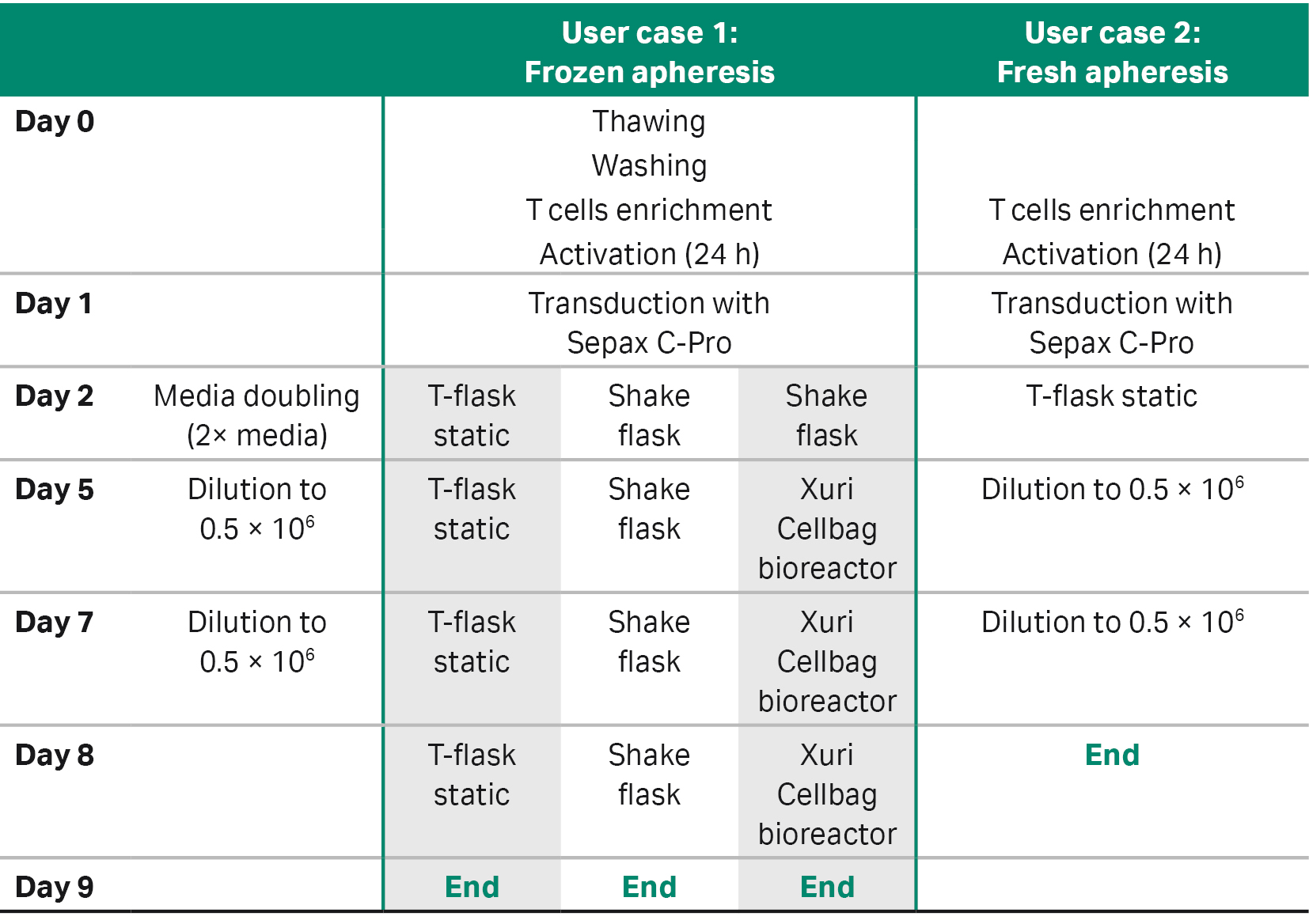
Table 1 outlines the options and ranges of parameters that users can customize to adapt the application software to their process. Here, we present two user cases as examples of using SpinOculation C-Pro application software for lentiviral vector transduction in different CAR T cell workflows (Fig 2).
Fig 1. SpinOculation C-Pro application software, combined with the Sepax C-Pro instrument and CT-60.1 single-use kit, offers a flexible, automated, and closed solution for T cell lentiviral transduction. This application can be incorporated into CAR T workflows that include a gene transfer unit operation.
Table 1. SpinOculation C-Pro application software specifications
Materials and methods: Automated and manual gene transfer using frozen and fresh apheresis units
User case 1: frozen apheresis
For the first user case, frozen apheresis units from three healthy donors were used. The frozen apheresis units were thawed using an automated dry thawing device*. The products were then washed using a functionally closed cell processing system† before initiating a manual T cell enrichment with the EasySep™ Release Human CD3 positive selection kit (STEMCELL Technologies). Isolated T cells were then manually activated in T-flasks (Thermo Fisher Scientific) with ImmunoCult™ Human CD3/CD28/CD2 T cell activator (STEMCELL Technologies) at a density of 1 × 106 cells/mL for 24 h in a humidified incubator (37°C, 5% CO2). Activated T cells were then transduced with LVV–enhanced green fluorescent protein (eGFP) (Tailored Genes) at a multiplicity of infection (MOI) of 2.5. The activated T cells were split into two groups for comparison transduction, in the traditional culture vessel method of Nunc™ Tissue culture-treated T-flasks, or transduced using SpinOculation C-Pro in the Sepax C-Pro instrument.
* For upstream thawing we used a proprietary device. An alternative would be to use the VIA Thaw™ instrument for both upstream and downstream processing.
†A functionally closed cell processing device was used in this study. However, the Sepax™ C-Pro instrument is recommended as the equivalent instrument for manufacturing purposes.
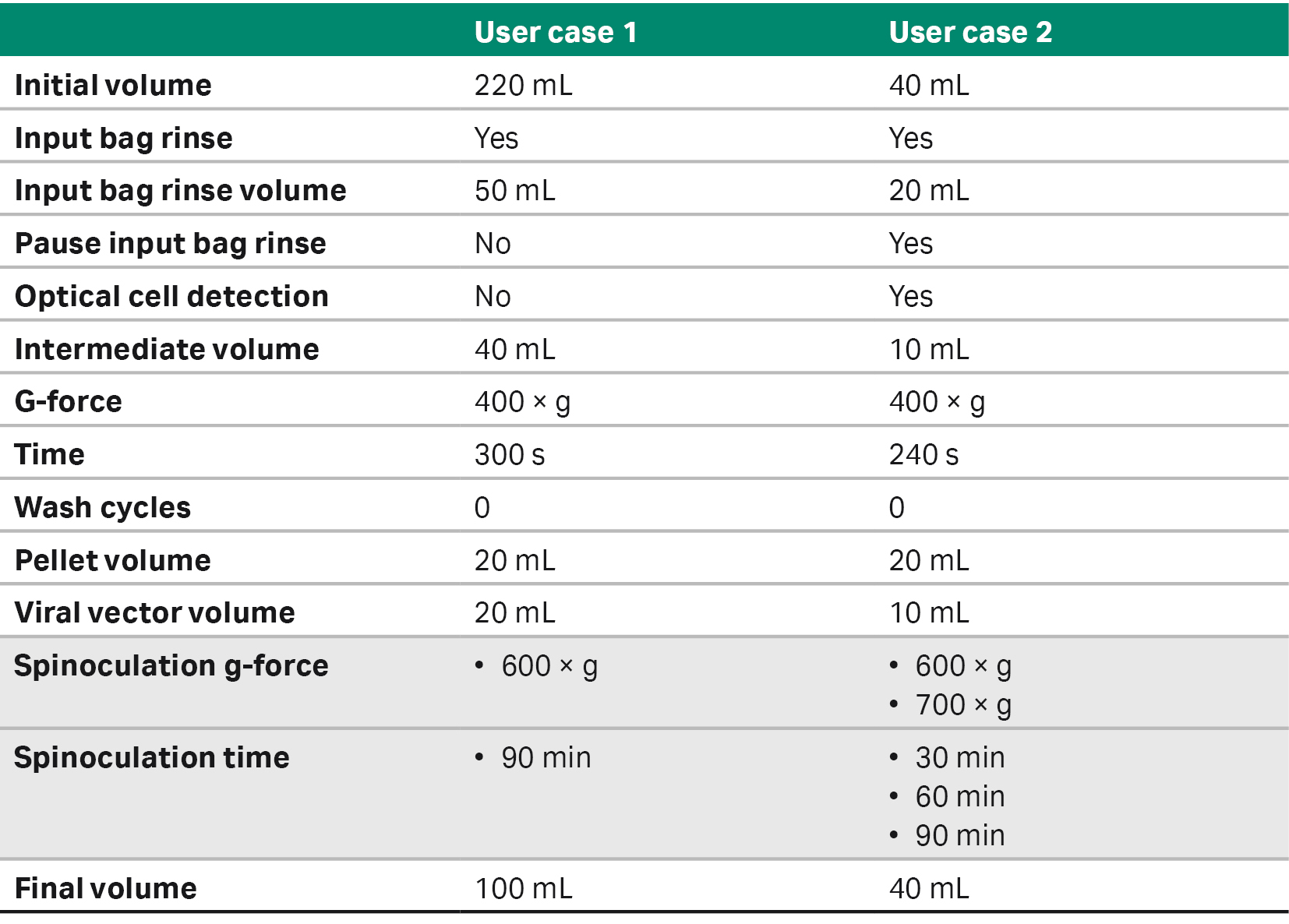
The traditional method included adding virus manually to the open culture flask, continuing through culture with fresh media added 16 to 20 h after transduction. Cells were transduced using SpinOculation C-Pro application software using a closed and automated method with virus contained within a sterile syringe (BD Biosciences) kept on ice. The cell-containing bag and virus-containing syringe with weldable lines were sterile welded onto the CT-60.1 Sepax C-Pro cell processing kit and run on the Sepax C-Pro instrument (Tables 2, 3).
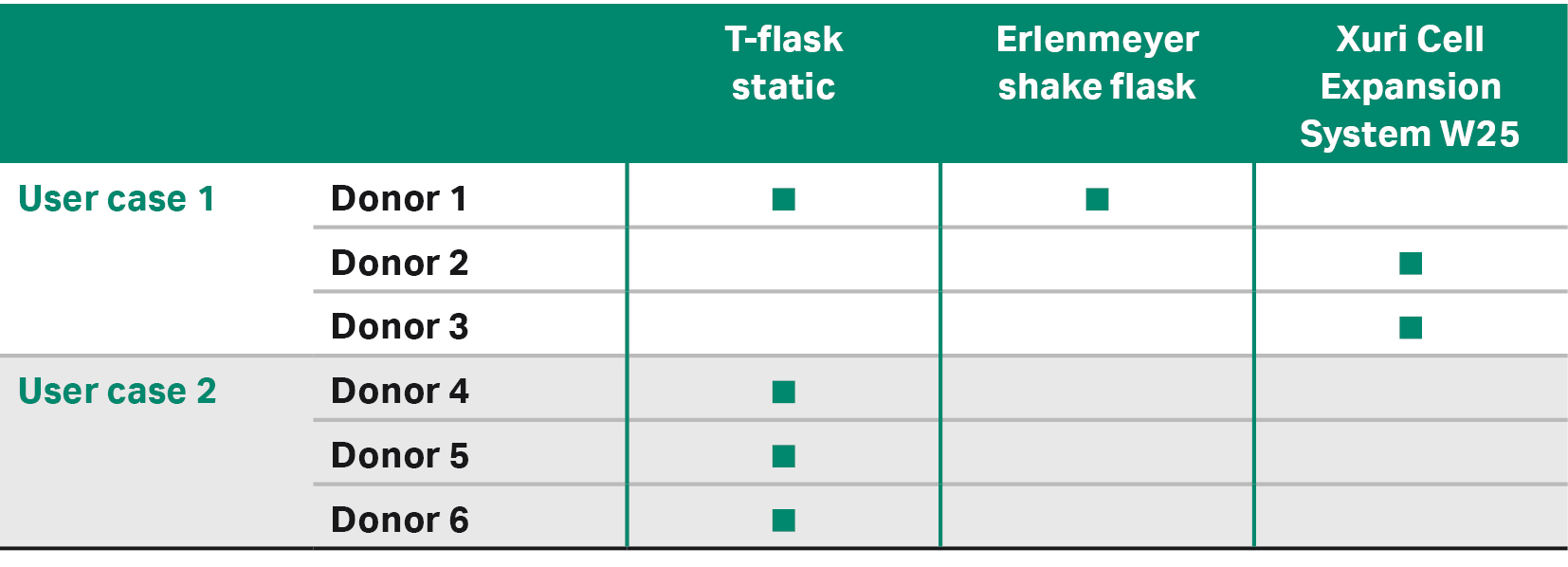
User case 1 compared three different downstream expansion methods of the transduced cells: two for low-scale expansion and one for large-scale expansion (Tables 3, 4).
The first method for low-scale expansion was similar to the manual culture described above, which expanded similarly to the spinoculation-transduced cells in the traditional and static Nunc tissue culture-treated T-flasks. The second method for low-scale expansion used a closed-vessel system and Erlenmeyer shake flasks that were seeded at a density of 1 × 106 cells/mL and placed on a MaxQ™ CO2 Plus Shaker at 90 rpm for dynamic culture, but still required placement within a humidified incubator (37°C, 5% CO2). These cultures continued for 3 d following transduction before being counted and diluted with fresh medium to 5 × 105 cells/mL on days 5 and 7. Cells were expanded until day 9 (8 d in total).
The third method was a large-scale expansion method. For the first 4 d, spinoculation-transduced cells were carried through the Erlenmeyer shake flask system and then expanded further in a 2 L Xuri™ Cellbag™ bioreactor (Cytiva). The Cellbag was placed on a Xuri Cell Expansion System W25 (Cytiva) filled with 5% CO2, and a reservoir containing culture medium was aseptically welded on. The medium was the same formulation used in small-scale culture. An initial volume of 200 mL culture medium was added to the bioreactor and allowed to equilibrate overnight. The system parameters were set at 37°C, gas flow rate to 0.05 L/min, and rocking rate to 10 rpm at a 6° angle. After a total of 4 d in the Erlenmeyer shake flask, culture was transferred and inoculated into the bioreactor and culture medium was added to a total volume of 500 mL of culture in the bioreactor. Once initiated, the bioreactors were left overnight while fresh medium was added to the reactor at 1 L/day to reach a total volume of 1 L. From day 5 onward, 1 L of medium/d was continuously perfused to control lactate, ammonium, and glucose levels.
Transduction efficiency was measured on the CytoFLEX™ Flow Cytometer (Beckman Coulter) 48 h after media addition. All subsequent steps were done using cell culture medium prepared by supplementing Xuri T Cell Expansion Medium (Cytiva) with 5% heat-inactivated human AB serum (Gemini) and 350 IU/mL Xuri IL-2 growth factor (Cytiva). Daily sampling was performed for cell number, viability, and biochemical analysis (lactate, ammonium, glucose, and pH).
At the end of the expansion phase (day 9), CAR T cells were cryopreserved using the VIA Freeze™ instrument (Cytiva). Flow cytometry analysis was performed at the end of expansion phase (day 9) to evaluate transduction efficiency. For large-scale experiments, after cell expansion, T cells were harvested using the Sefia™ S-2000 Cell Processing instrument, FlexCell application software, and CT-800.1 Cell Processing kit (Cytiva). Briefly, 1 × 1010 T cells were reduced in volume to 50 mL at a 75 mL/min flow rate. Two wash cycles were performed using PLASMA-LYTE A (Baxter) supplemented with 10% human serum albumin (Gemini). Washes were performed at 400 × g for 5 min. T cells were then reformulated at 1 × 108 cells/mL in PLASMA-LYTE A with 50% CryoStor™ CS10 (BioLife Solutions) and 5% human serum albumin into three CryoMACS™ 50 freezing bags (Miltenyi Biotec). The three cryogenic bags were loaded into the VIA Freeze Quad™ freezer (Cytiva) and frozen at a cooling rate of -1°C/min until the temperature reached -100°C. After freezing, all cells were transferred to liquid nitrogen storage.
User case 2: fresh apheresis
For the second user case, fresh apheresis units from three separate healthy donors were used. The first step consisted of a manual T cell enrichment with the EasySep Release Human CD3 positive selection kit (STEMCELL Technologies). Isolated T cells were then manually activated in T-flasks (Thermo Fisher Scientific) with ImmunoCult Human CD3/CD28/CD2 T-cell activator (STEMCELL Technologies) at a density of 1 × 106 cells/mL for 24 h in a humidified incubator (37°C, 5% CO2). Activated T cells were then transduced with LVV–eGFP (Jiman Biotech) at an MOI of 5. A portion of activated T cells was transduced in Nunc tissue culture-treated T-flasks, and culture media volumes were doubled with added fresh media 24 h after transduction. The other portion of activated T cells was transduced using SpinOculation C-Pro application software. The cells and the viral vector that were supplied in a bag were welded onto the CT-60.1 Sepax C-Pro Cell Processing kit and run on the Sepax C-Pro instrument (Tables 2, 3). Transduction efficiency was measured on the flow cytometer (BD Canto) 48 h after media addition. All subsequent steps were done using cell culture medium prepared by supplementing RPMI 1640 (Gibco) with 10% fetal bovine serum and 250 IU/mL Xuri IL-2 growth factor (Cytiva) (Table 4).
User case 2 used only one static expansion method (Table 3). Transduced cells were inoculated into Nunc tissue culture-treated T-flasks and seeded at a density of 1 × 106 cells/mL. Cells were cultured in an incubator (37°C, 5% CO2) for 3 d before being counted and diluted to 5 × 105 cells/mL. Cells were maintained in culture for an additional three days and then (day 8, at the end of expansion phase) phenotypic analysis was performed by flow cytometry to evaluate transduction efficiency.
Table 2. SpinOculation C-Pro application software parameters for each user case
Table 3. Expansion methods used for each donors and user cases
Table 4. Detail of processes workflows
Results: Automated versus manual CAR T cell lentiviral transduction by spinoculation
Comparison of transduction efficiency obtained with automated procedure versus respective manual controls
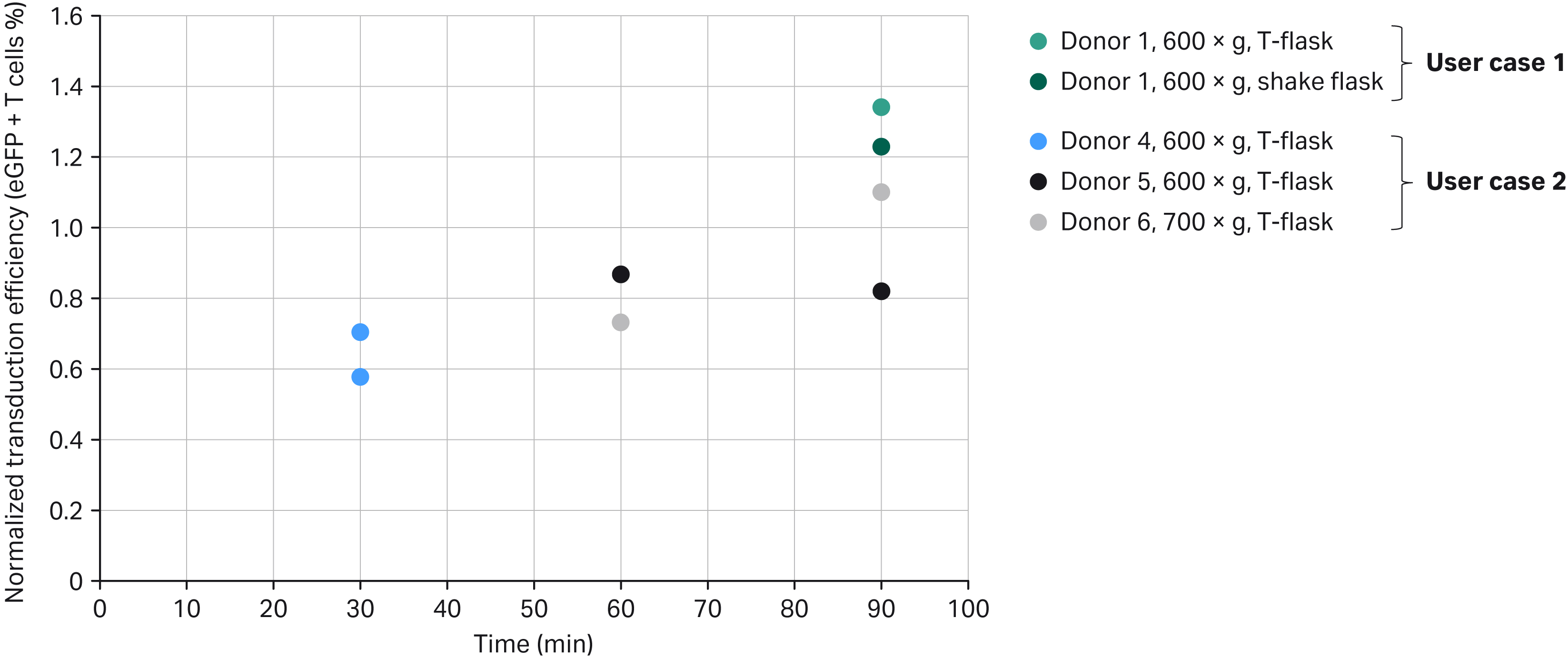
Fig 3. Normalized transduction efficiency of SpinOculation C-Pro application versus manual performance at end of expansion phase. Note that Donors 2 and 3 are not represented in this graph because they were done only in the Xuri Cell Expansion System W25 without a T-flask control group (see also Table 3 and Fig 5).
Transduction efficiencies from four donors (one from User case 1 and three from User case 2) were normalized by comparing the different spinoculation processes to their respective split donor static control arm, which was manually processed in an open T-flask (User case 2 and one of the two User case 1 data points) or in agitated Erlenmeyer shake flasks (second user case, one data point). Donor 1 material was processed using the User case 1 workflow in a nine-day process (24 h for activation and 8 d of cell expansion). Donors 4, 5, and 6 materials were processed using the User case 2 workflow in an eight-day process (24 h for activation and 7 d of cell expansion).
Transduction efficiencies were evaluated every two days. Figure 3 represents the transduction efficiency obtained at the end of the expansion phases (day 9 for User case 1 and day 8 for User case 2). By increasing spinoculation time from 30 to 90 min in the Sepax C-Pro, we observed that transduction efficiency at the end of the expansion phase tended to increase with some variability between donors.
A large range of transduction efficiencies, between 31% and 75%, was observed. The transduction efficiency might depend on multiple factors, like the viral construct source, the workflow used (User case 1 versus User case 2), the combination of protocol parameters selected, and the donor material.
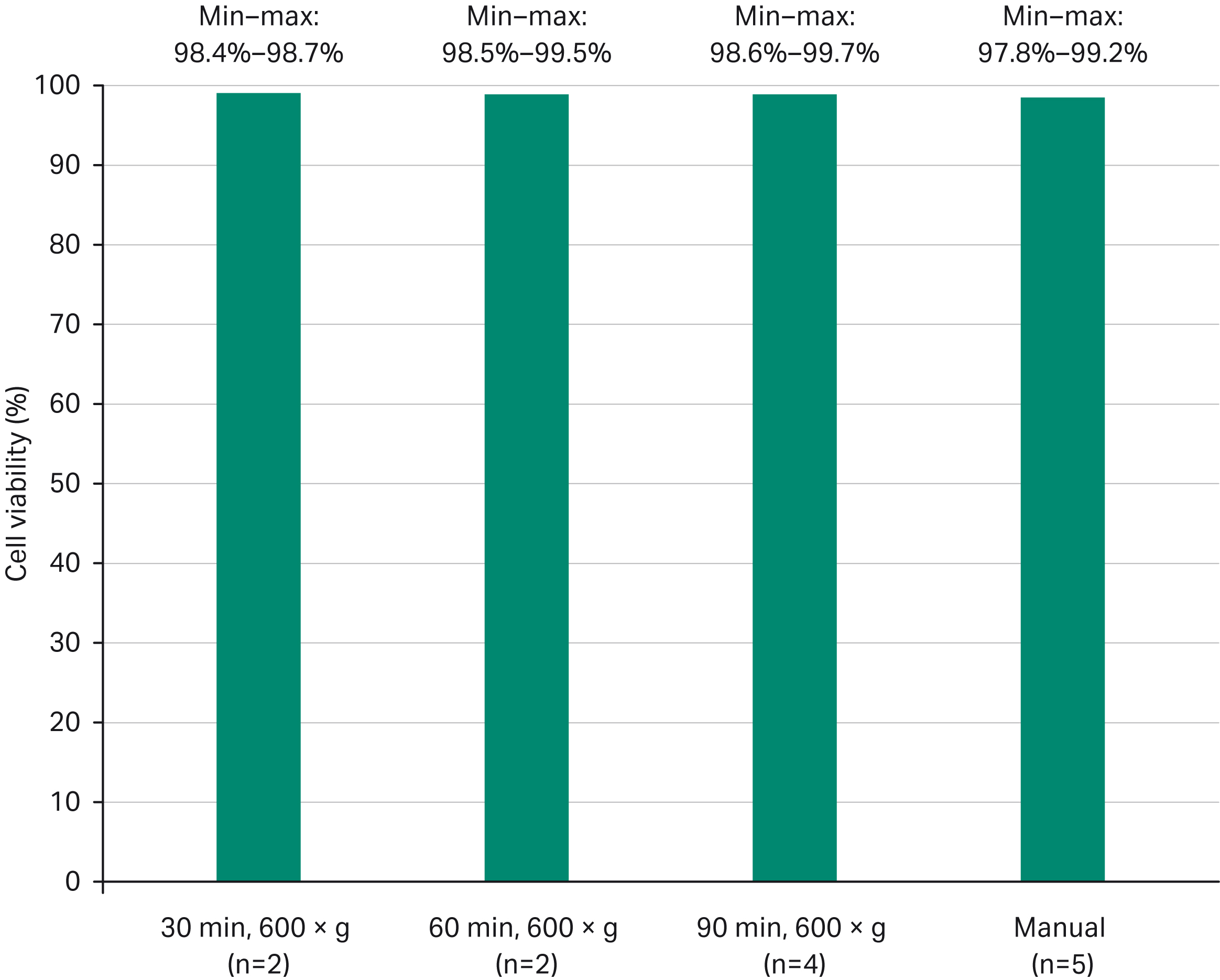
Fig 4. Average cell viability (%) obtained at the end of the expansion phase after manual versus automated processes.
Despite cell inoculation with LVV for 90 min at high g-force (700 × g), the cell viability was maintained above 97% during the subsequent expansion phase in all cases and was comparable to the manual process.
Comparison of transduction efficiency obtained with User case 1 and different expansion methods
Figure 5 compares the transduction efficiency obtained after 8 d of cell expansion using three different expansion methods. The four experiments were done using the User case 1 materials and methods. For the manual methods (T-flask and shake flask), input product came from the same donor. For the two experiments done using the automated Xuri Cell Expansion System W25, input product came from two distinct donors.
Fig 5. Transduction efficiency (%) obtained after spinoculation at 600 × g during 90 min in Sepax C-pro followed by 8 d of expansion using manual (T-flask, shake flask) or automated (Xuri Cell Expansion System W25) processes.
When using the method from User case 1 and the corresponding LVV MOI of 5, transduction efficiencies at the end of the expansion phase were above 70% across all experimental conditions, including varying biological donors and expansion methods.
We observed that when using input material from the same donor (Donor 1) across different expansion methods (T-flask versus shake flask), transduction efficiencies obtained at the end of expansion were comparable (75% and 74%, respectively); when using the same expansion method (Xuri Cell Expansion System W25) but distinct donor materials (Donors 2 and 3), the transduction efficiency obtained was between 82% and 89% at the end of expansion (Fig 5).
Conclusions: Closed, automated LVV transduction in CAR T cell therapy manufacturing
As these user cases demonstrate, SpinOculation C-Pro application software offers a solution for a closed, automated LVV transduction step where transduction efficiency can be comparable to a manual, open process. The open software parameters provide a wide range of flexible options for process development and optimization by allowing users to vary multiple parameters (Table 1).
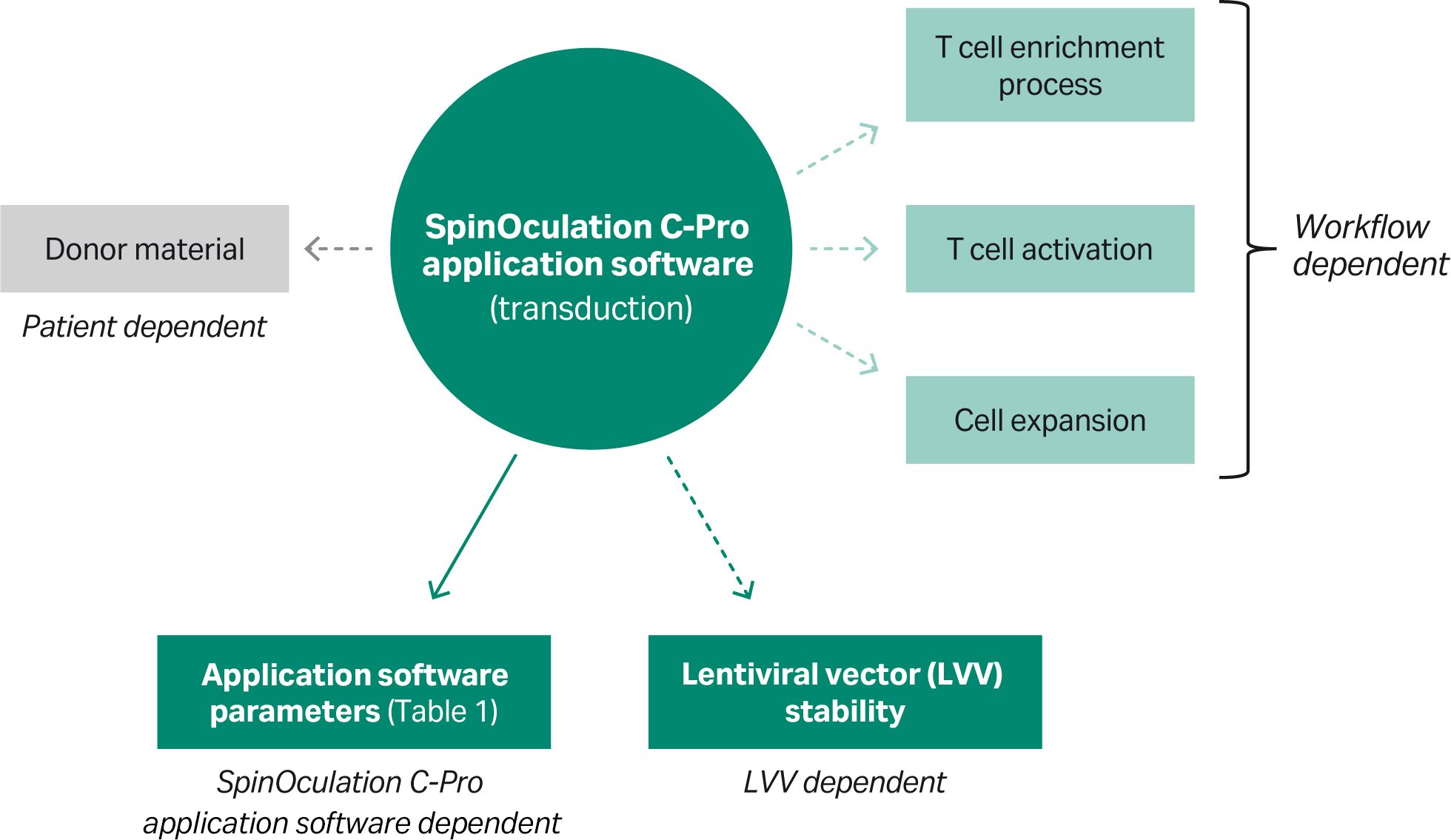
It is important to consider that many factors influence the performance that can be achieved (Figs 3, 4). Notably, however, transduction efficiency was shown to be highly dependent on LVV source and the MOI used. Moreover, the upstream preparation of T cells (enrichment phase) as well as the method and reagents used to expand transduced T cells might impact transduction levels (Figs 5, 6). Unit operations and processing steps downstream of transduction, such as T cell expansion methods and culture vessels, can also affect the transduction efficiency of the final therapeutic product (Fig 5).
The SpinOculation C-Pro application software addresses the need for a closed and automated workflow solution for CAR-T cell therapies, helping to standardize processes, minimize risk, and reduce variability.
Fig 6. A simple solution for a complex model. Overview of factors that could influence transduction performance. While most factors can be standardized, the donor material is subject to variability.
* For upstream thawing, we used a proprietary device. An alternative would be to use the VIA Thaw™ instrument for both upstream and downstream processing.
† A functionally closed cell processing device was used in this study. However, the Sepax C-Pro instrument is recommended as the equivalent instrument for manufacturing purposes.