Freezing cells for cryopreservation
Successful cell cryopreservation requires a controlled cooling rate. Cooling too rapidly or too slowly will lead to a poorer outcome. This is true because of the physical environment cells experience during cryopreservation. As ice forms in the extracellular solution, pure water is locked away as ice. As a result, cells are suspended in an increasingly concentrated solution, which dehydrates them. Cooling too slowly makes this solution toxic; cooling too rapidly prevents cells from dehydrating sufficiently, and intracellular ice forms.
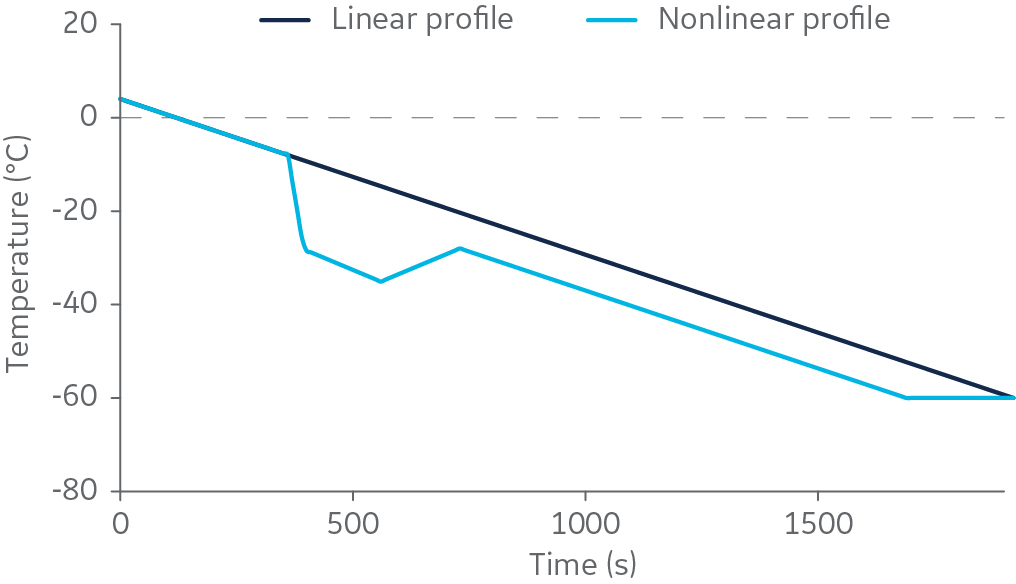
Controlled-rate freezers, such as the electric-powered VIA Freeze series or liquid nitrogen (LN2)-based freezers, are often programmed for linear cooling. A subset of researchers using LN2-based freezers prefer to interrupt the linear cooling to include a “rapid-cooling nucleation step,” which they believe is needed for successful cryopreservation. This step is hypothesized to improve ice nucleation – an important event for good post-thaw outcomes. A typical cooling profile for linear cooling with a rapid-cooling nucleation step is shown in Figure 1.
Fig 1. A linear 2°C/min cooling profile compared with a typical profile that includes a rapid-cooling nucleation step (1).
This study explores how a rapid-cooling nucleation step affects viable cell count and activity of four cell lines. Three conditions were tested: linear-rate freezing in a VIA Freeze system; linear-rate freezing in an LN2-based freezer; and linear-rate freezing in an LN2-based freezer, interrupted by a rapid-cooling step.
Experimental design
Cells in cryovials (1 mL fill volume) were cooled at 2°C/min from 4°C to -60°C in two controlled-rate freezers – VIA Freeze system (Cytiva) and a conventional LN2-based freezer (Kryo 560, Planar). A third freezing method in this same LN2-based freezer incorporated the rapid-cooling nucleation protocol from Table 1. Five cryovial samples, with 10% DMSO as cryoprotectant, were used for each condition. In all experiments, samples were plunged into LN2 when the temperature reached -60°C. The cryovials were stored below -140°C for at least 24 h, and then thawed. Thawed cells were placed into cell culture medium and allowed to grow in a standard humidified CO2 incubator at 37°C.
Table 1 lists the corresponding cooling rate sequence that was programmed into the LN2-based controlled-rate freezer.
Table 1. Programmed parameters for experiment that includes rapid-cooling nucleation step.
| Step | Cooling rate | Temperature range |
| 1 | -2°C/min | 4°C to -8°C |
| 2 | -35°C/min | -8°C to -28°C |
| 3 | -2.5°C/min | -28°C to -35°C |
| 4 | +2.5°C/min | -35°C to -28°C |
| 5 | -2°C/min | -28°C to -60°C |
Four cell lines were examined: Chinese hamster ovary (CHO); HepG2 (human liver); MG-63 (human bone); and Jurkat (human suspension immune) cells. Viable cell count after thawing was determined using a Cytell Cell Imaging System (GE). An alamarBlue™ bioassay was performed using a Tecan™ plate reader to evaluate metabolic function of cells after 3 h incubation. These functional tests were carried out at 24, 48, and 72 h of culture post-thaw.
Results from different freezing methods
Figure 2 shows no significant difference in post-thaw results between HepG2 cells cooled in a LN2-based freezer with or without a rapid-cooling nucleation step included. Furthermore, there is no significant difference in results using either freezer system and a linear cooling rate. The same pattern was observed with CHO cells (Fig 3), as well as Jurkat and MG-63 cells (data not shown, to keep the article brief).
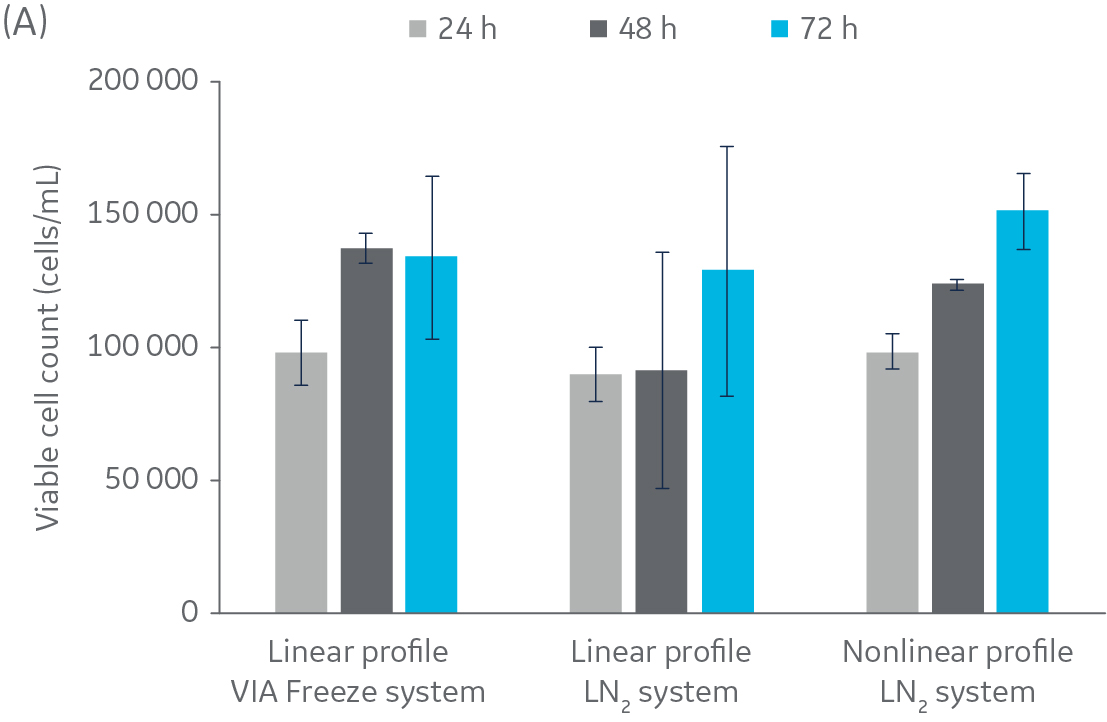
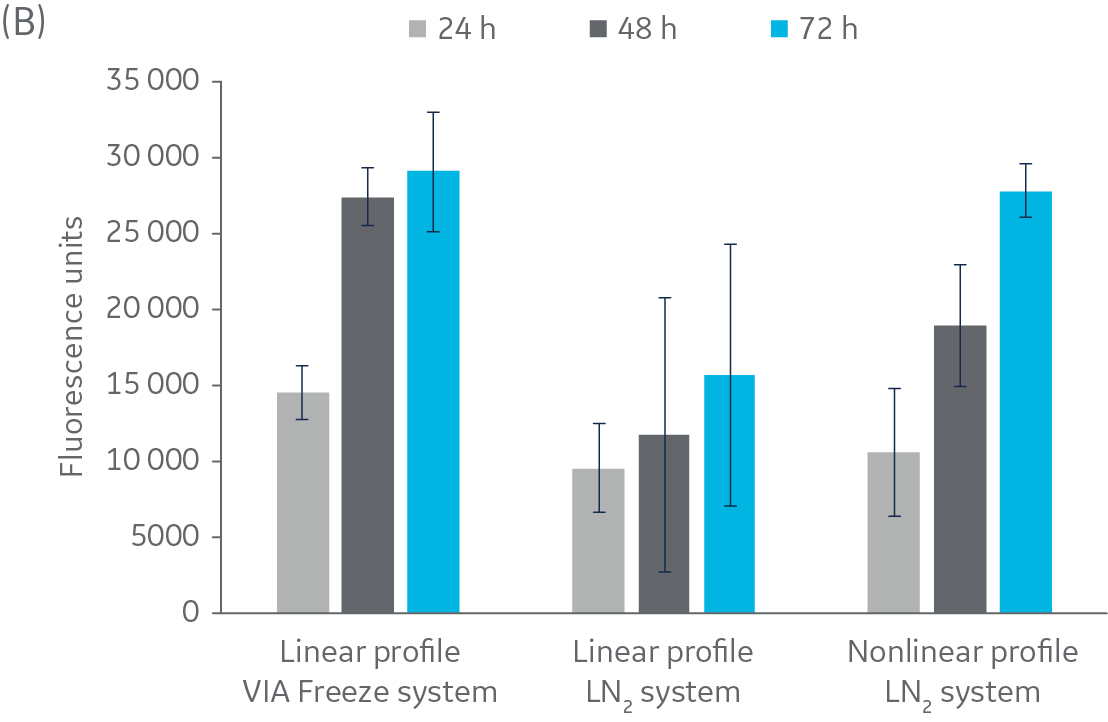
Fig 2. Post-thaw results for HepG2 cells. Cryovials were assessed 24, 48, and 72 h of culture post-thaw. (A) viable cell count, and (B) alamarBlue functionality. n = 5 ± SD.
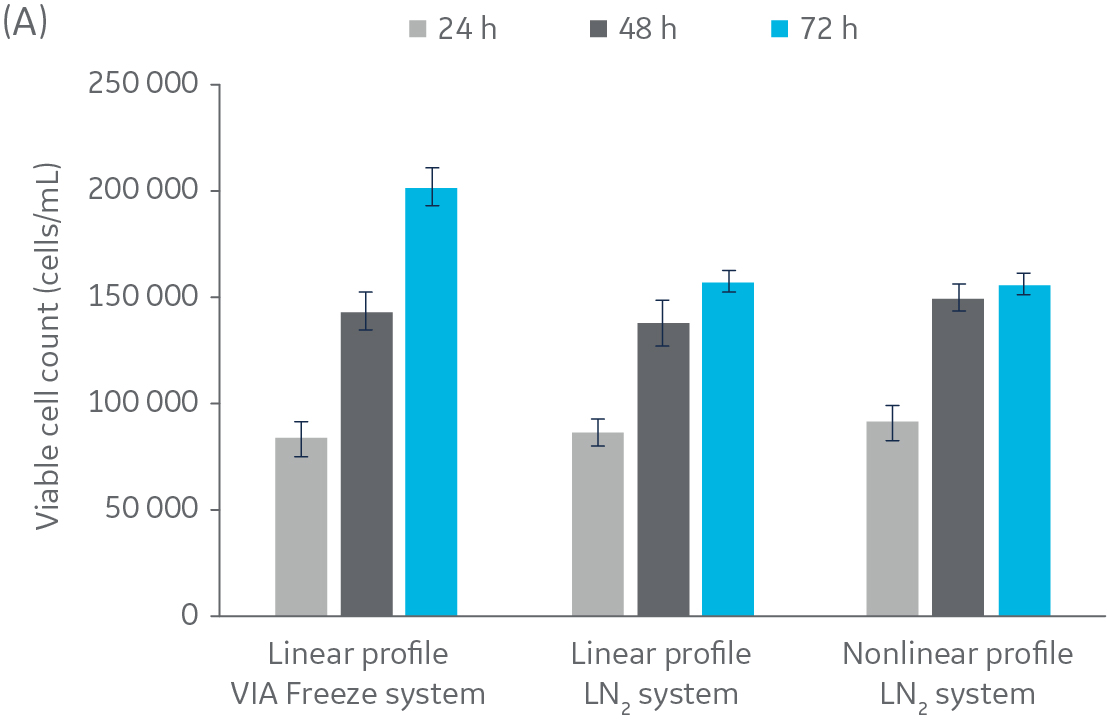
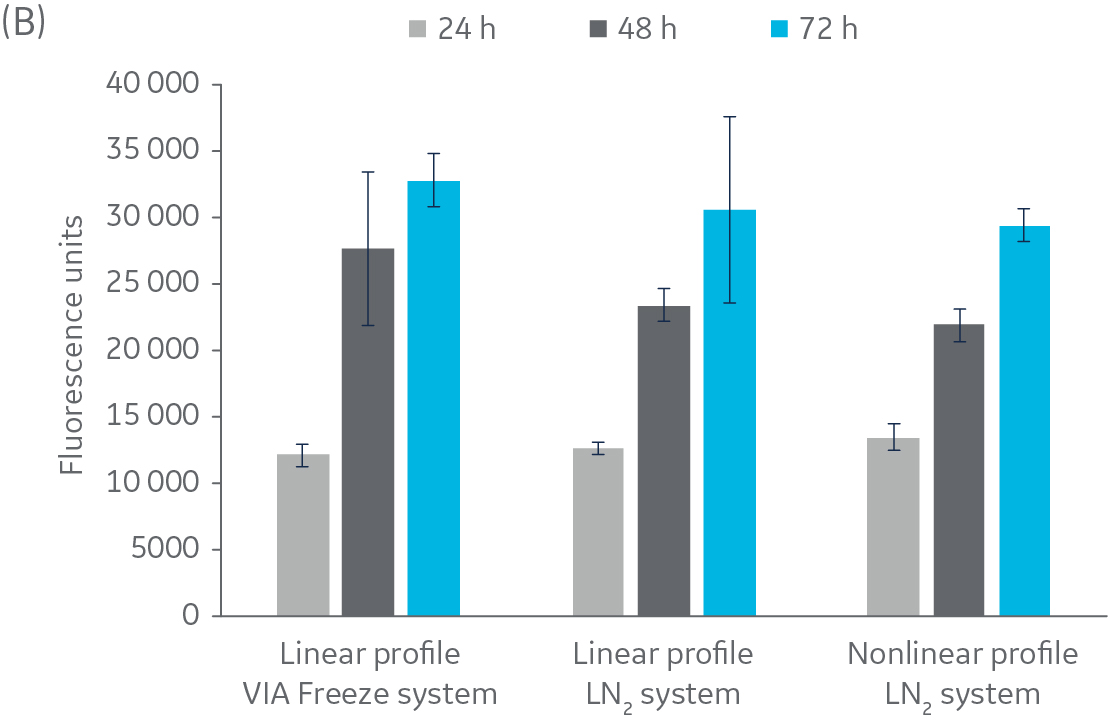
Fig 3. Post-thaw results for CHO cells. Cryovials were assessed 24, 48, and 72 h of culture post-thaw. (A) viable cell count, and (B) alamarBlue functionality. n = 5 ± SD.
Conclusions
In this study, including a rapid-cooling nucleation step in LN2-based freezer did not improve post-thaw results for the four tested cell lines. No literature was found to support the hypothesis that a rapid-cooling step to induce ice nucleation is beneficial for cryopreservation.
Using VIA Freeze system for freezing cells
In addition to demonstrating that a rapid-cooling step is unnecessary, the study shows comparable results using LN2-based and VIA Freeze systems programmed with a linear cooling rate. Although cell viability and function are expected to be similar, the VIA Freeze system offers several advantages over LN2-based controlled-rate freezers. Liquid nitrogen is not used for cooling, which avoids the associated contamination risks and supports GMP compatibility. Furthermore, using a VIA Freeze system is expected to reduce operating costs.
Reference
Read about cryopreservation for cellular therapies in The Big (Bio) Freeze article.
Learn how VIA Freeze systems can help you effectively manage your cryochain.