In this study, a team of scientists from Cytiva and CCRM developed a scalable process to produce high-yield lentiviral vector batches using stable, suspension-based production methods.
Key takeaways:
- Current lentiviral vector production methods are expensive, labor-intensive, and difficult to scale.
- The team addressed these issues with a new manufacturing workflow that includedsuspension-based culturing and a shift from transient to stable transfection.
- This led to a simple batch process that does not use animal-derived components. The process is scalable from research to production in single-use, stirred tank bioreactors.
Trailing behind
After decades of basic research and small academic trials, cell and gene therapies have officially arrived. Several programs have secured Food and Drug Administration (FDA) and European Medicines Agency (EMA) approval, including several ambitious CAR T cell therapies. And many more are on the cusp of commercialization. But for now, large-scale manufacturing techniques and infrastructure are trailing behind.
One critical and expensive raw material for cell and gene therapy manufacturing is the viral vectors used to introduce new genetic material into the cell. The production of viral vectors has been constrained by current manufacturing methods, which are manual and limited in scale. This has caused lentiviral vector (LV) demand to rapidly outpace supply.
The status quo can support small exploratory studies, but it is insufficient for late-stage trials and commercialization. New, scalable, efficient, and robust viral production processes are urgently needed to support immunotherapy and gene therapy commercialization.
With this in mind, we developed a new manufacturing workflow, addressing two technical challenges that have limited the efficiency and scalability of LV production. The robust process uses a suspension-adapted producer cell line, and LV production was achieved in short and simple batch processes. Production was successfully scaled in a linear manner from 5 L to 28 L in single-use, stirred-tank bioreactors.
Limited scalability with adherent cultures
For small-scale production, lentiviral vectors are typically produced using adherent HEK293 cell lines in serum-containing media. With manual adherent culture methods, cells are grown as a single layer on a substrate designed for cell adhesion and spreading. This is slow and labor-intensive, and the use of serum-based media can trigger an immune response in vivo. Above all, adherent cell culture methods are difficult to scale to meet the needs of a growing industry; viral production is tied to available surface area, which in turn constrains product yield.
To overcome these limitations, several academic and industrial laboratories have been working to develop a suspension-based production process. Suspension cultures have several advantages compared to adherent methods. For example, they are scalable from a few milliliters to hundreds and thousands of liters. Also, the cultures are more amenable to fully closed processes that can be automated. These strategies are a critical step towards reducing the cost and time required to generate high yield, clinical-grade LV for commercial operations.
In this study, we used a suspension-adapted cell line capable of growth in serum-free conditions, and we optimized feeding strategies and conditions in stirred-tank bioreactors. As a result, cell growth and viral production were improved. A key element for the successful generation of this stable producer cell line, clone 92, was the use of a double switch system. Using this system two inducers are required to initiate the synthesis of LV, which offers a tight control on the production of LV cytotoxic elements (1).
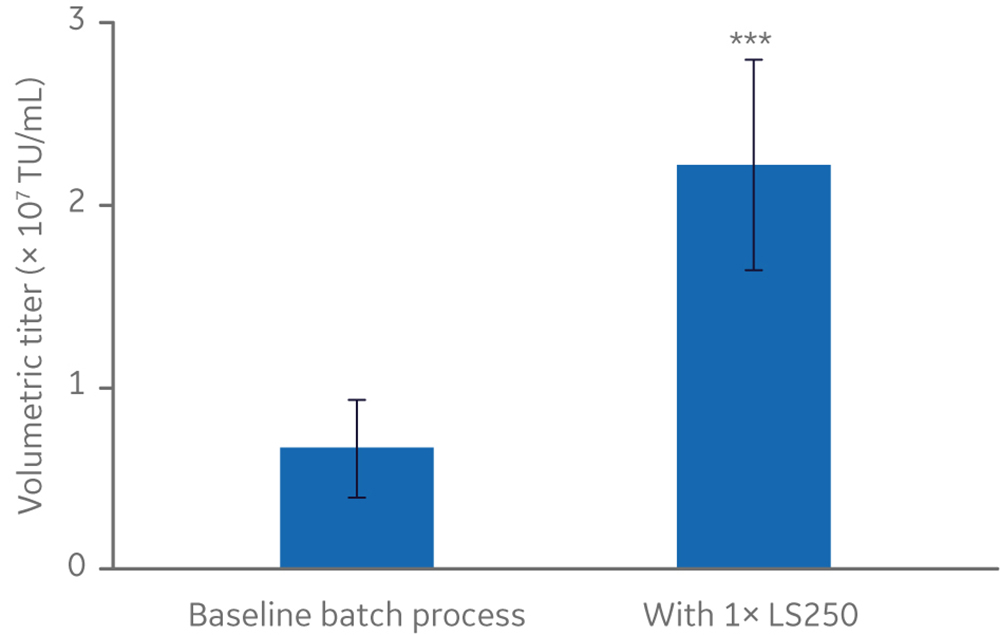
One example of the improvements in yield was a more than three-fold increase in LV titers in a batch production process with the addition of a lipid-based supplement (Fig 1). This, coupled with additional optimization, led to the successful demonstration of LV production in an Xcellerex 50 L single-use bioreactor.
Fig 1. HyClone LS250 lipid supplement boosted yields in shake flasks.
Low transfection efficiencies using existing techniques
A second major barrier to large-scale LV production is the reliance on multi-plasmid transient transfection methods to introduce new genetic material. For transient transfection, the vector and transgene plasmids are introduced post-expansion. While versatile, this approach is costly at larger scales due to expensive raw materials. It is also prone to contamination and can be more variable than using a stable producer line.
Although inducible stable expression cell lines require a significant upfront investment of time and money, long term they are simpler and less costly to scale up. This makes them an attractive solution for commercial-scale manufacturing — provided the same LV yields can be achieved. One of the key challenges here is ensuring that the DNA is efficiently transfected and that the successful cells can be identified and isolated.
In this study, we used a stable producer cell line, derived by stable transfection from a suspension cell packaging cell line. Throughout the process, we optimized the induction conditions and feeding strategies to further improve viral production without increasing costs. For example, a series of studies were carried out to determine at which point in the culture to induce viral production. Ultimately, by driving up viable cell concentration, the total number of infectious viral particles was increased. This generated yields more akin to less scalable methods, but with all the benefits of stable transfection.
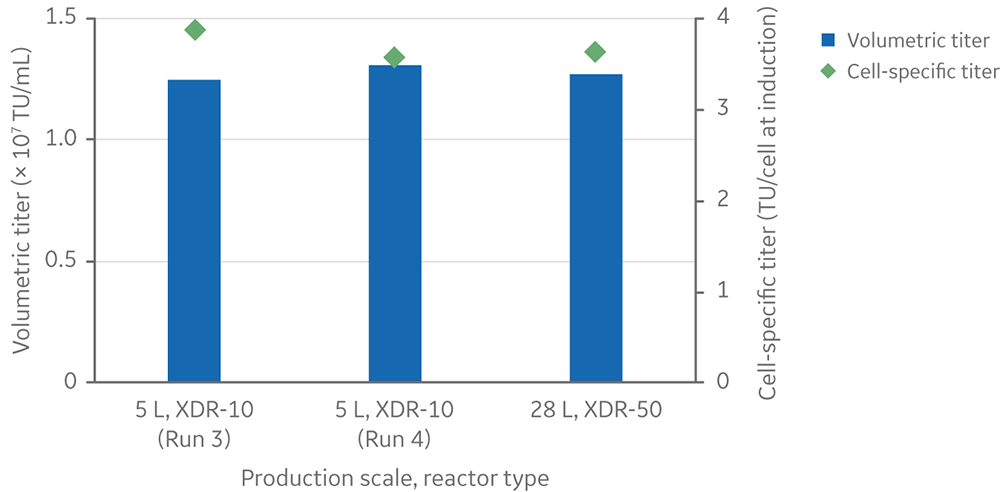
The developed process, with small adjustments, was also shown to be scalable to 28 L in a 50 L stirred-tank bioreactor system (Fig 2).
Fig 2. Lentiviral vector titers for 28 L run compared with two 5 L runs.
Summary
Importantly, we were able to draw on existing biomanufacturing expertise and bioprocessing technology to produce consistent batches that scale up in a linear manner to 28 L.
Outcomes:
- Improved output: The methods used are readily scalable, enabling commercial-scale production of LV.
- Consistency: The closed process described was reproducible and robust.
- Cost reduction: Along with improved viral production, automation, and avoiding expensive raw materials needed for transfection-based methods, the use of serum-free alternatives reduces the need for expensive post-infusion immunosuppressants.
This study provides a new benchmark for scalable, high-quality LV production. Manufacturers must continue to raise the bar to meet global demand for approved and soon-to-be-approved cell and gene therapies.
For details, read about an upstream lentiviral vector process that can be adapted for GMP compliance.
Acknowledgements
We gratefully acknowledge the National Research Council of Canada for technical guidance and providing the stable inducible LV producer lines used in these studies.
All work was performed in collaboration with CCRM through funding from FedDev Ontario and Cytiva at the Centre for Advanced Therapeutic Cell Technologies (CATCT), Toronto, Ontario, Canada. The reporting and interpretation of the research findings are the responsibility of the author(s).
References
- Manceur, A. P. et al.Scalable lentiviral vector production using stable HEK293SF producer cell lines.Hum. Gene Ther. Methods28, 330–339 (2017).