By Federico Saltarin, Marie-Laure Collignon, Martina Reina, Pankaj Salvi, Zi Ying Tan
Background and introduction
The iCELLis Nano and iCELLis 500+ bioreactors are single-use, fixed-bed bioreactors that are capable in scaling up adherent cell culture processes, commonly used for gene therapy applications such as adeno-associated virus (AAV) and lentivirus vectors. Due to the different compactions of the proprietary macrocarriers and the fixed-bed heights, the macrocarriers provide a broad range of cell growth surface area ranging from 0.53 m2 in the iCELLis Nano bioreactor to a maximum of 500 m2 in the iCELLis 500+ bioreactor. Batch processes are the most straightforward type of processes that can be run in the iCELLis bioreactor range, where the bioreactors are filled with cell culture medium and cells to a defined working volume and no additional medium is added. In batch processes, cells would gradually consume the nutrients from the cell culture medium, and cell metabolites and inhibitors would accumulate in the cell culture medium over time. Therefore, this approach is limited in time, depending on the rate of nutrients consumption and metabolites accumulation. Furthermore, an optimized process transferred from flatware might require a working volume that exceeds the maximum working volume in the iCELLis bioreactors, hence imposing an additional restriction to batch processes. To address these limitations, two alternative processes are possible in the iCELLis bioreactors: recirculation and perfusion.
Recirculation
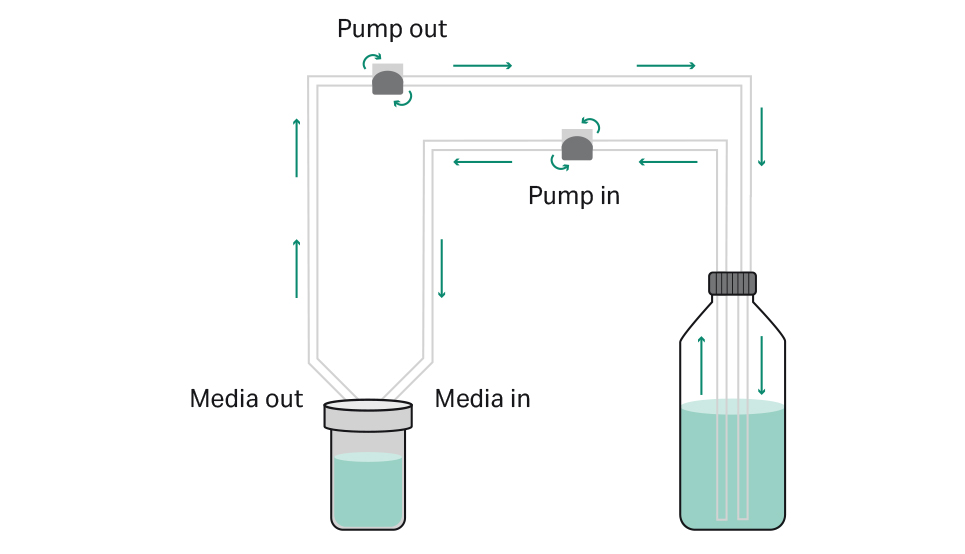
When the total working volume required by the specific process is higher than the maximum volume in the bioreactor (e.g., greater than 850 mL in the iCELLis Nano bioreactor), a recirculation manifold – an external bottle (or container) filled with the additional required volume of media – can be connected to the bioreactor. Two separate tubings of the recirculation manifold are connected to the ‘media in’ and ‘media out’ lines respectively and inserted into peristaltic pumps that operate from the software interface of the iCELLis bioreactor. Through the activation of the pumps, media recirculates at a defined flow rate between the bioreactor and the external bottle, providing the adherent cells with the total required amount of cell culture medium and nutrients. This approach can extend a typical batch process by providing greater volume of media. Nevertheless, similar to the classical batch process, glucose and nutrient concentrations decrease over time and metabolic by-products (e.g., lactate, ammonia) accumulate in the culture medium. A schematic view of the recirculation process in the iCELLis Nano bioreactor is shown in Figure 1.
Fig 1. Schematic view of recirculation process. The recirculation manifold (right side) is connected to the iCELLis Nano bioreactor (left side). The circular arrows show the rotary direction of the peristaltic pumps. The straight arrows indicate the direction of media flow during recirculation: medium recirculates continuously between the bioreactor and the bottle.
Perfusion
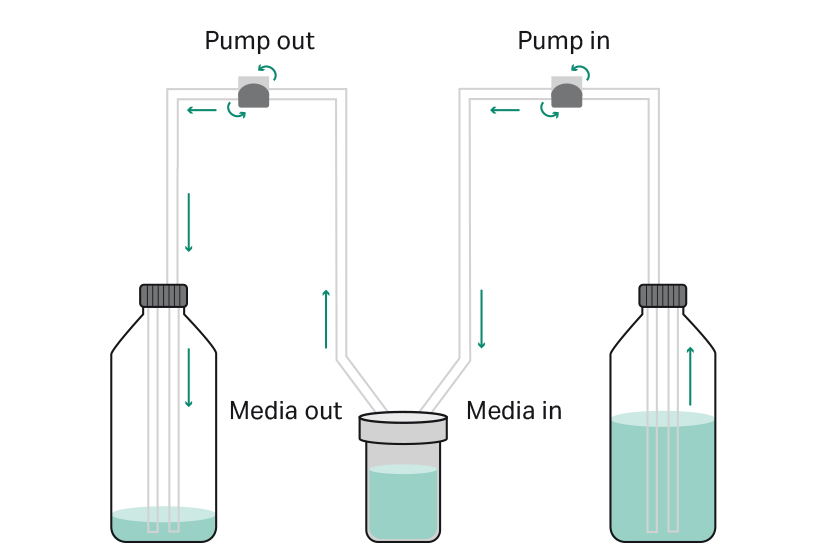
Perfusion boosts biomanufacturing processes by maintaining cells in a steady state with potentially higher viable cell densities and product titers. A bottle with fresh medium is connected to the ‘media in’ line and continuously delivers fresh media. A second empty bottle (or container) is connected to the ‘media out’ line and collects spent media out of the bioreactor. Essential nutrients (e.g., glucose) are continuously replenished and toxic metabolites or products (e.g., lactate, viral particles) are continuously removed and harvested from the bioreactor. The aim of this approach is to achieve homogeneous cell culture media composition (nutrient and metabolite concentrations), while maintaining a constant total working volume. Perfusion provides optimal growth conditions to the cultured adherent cells while simultaneously harvesting possibly unstable products such as viral vectors. Perfusion can be implemented at different phases of the process to achieve different objectives. In the growth phase, relatively higher cell numbers can be achieved by perfusion as this circumvents nutrients limitation and reduces the inhibitory effect of waste metabolites accumulating in the culture media. This enables the cells being maintained in a healthy steady state for subsequent transfection and production.
Alternatively, perfusion can be implemented in the production phase for products such as budding viral vectors e.g., lentivirus or secreted recombinant proteins. Perfusion process in the production phase facilitates the harvest of product continuously or in lots. This benefits in improved recovery of less stable or aggregation-prone products during downstream processing. This may also benefit the culture by extending the duration of the production phase resulting in improved productivities compared to a batch process. Perfusion can also be performed to exchange media and remove serum or polyethyleneimine (PEI) from the bioreactor. Figure 2 shows a schematic view of the perfusion process in the iCELLis Nano bioreactor.
Fig 2. Schematic view of a perfusion process. The external bottle containing fresh medium (right side) is connected to the iCELLis Nano bioreactor ‘media in’ line (center, right). The empty (harvest) bottle (left side) is connected to the iCELLis Nano bioreactor ‘media out’ line (center, left). The circular arrows show the rotary direction of the peristaltic pumps. The straight arrows indicate the direction of media flow during perfusion: fresh media is continuously pumped into the bioreactor whereas consumed media is continuously pumped out of it.
The choice between batch, recirculation or perfusion process depends on the characteristics of the specific cell culture and product. Furthermore, strategic assessment may influence this decision. For example, recirculation can be a faster way to market, whereas perfusion may require further optimization despite the potential superior process. Hence, recirculation can be applied in the initial development phase up to the clinical trials, and perfusion can be explored during process development. Table 1 summarizes the main advantages and challenges of recirculation and perfusion processes.
| Recirculation advantages | Recirculation challenges | Perfusion advantages | Perfusion challenges |
| Extended working volume compared to a batch process. | Media composition is not homogeneous throughout the process, leading to build-up of toxic by-products. This can be overcome with media exchange. | Initiate earlier harvest of viral vectors that are not stable in bioreactors | Longer process development and optimization. |
| Straightforward technology transfer by maintaining the same media volume to surface area ratio from flatware processes. | Aimed to achieve homogenous media composition throughout the perfusion process. | Larger working volumes in some cases, which may increase downstream processing. | |
| Faster process development. | Better metabolite profiles in the bioreactor, such as glucose and lactate concentrations | ||
| Can reduce equipment and facility footprint if stainless steel mixers are replaced with single-use bags. |
Table 1. Advantages and challenges of recirculation and perfusion processes.
In this application note, we will illustrate the scientific rationale of recirculation and perfusion processes, providing methodical considerations and guidance on how to perform and optimize these processes in the iCELLis bioreactors.
Considerations for recirculation
For customers considering the recirculation approach, the first step involves calculation of total working volume and the volume in the recirculation bottle. The media volume to surface area (V/S) ratio of the flatware process is typically kept the same when the process is transferred to the iCELLis bioreactor as a proof-of-concept. Indeed, maintaining the same ratio would result in similar cell culture metabolism in the bioreactor and the flatware.
The total working volume and the volume in the recirculation bottle are computed using the formula below and a practical example is explained in the next paragraph: If 35 mL of media is added in a T-175 flask (175 cm2), the V/S ratio is 0.2 mL/cm2. Table 2 shows the total working volume required for each size of the iCELLis Nano bioreactor to maintain the same 0.2 mL/cm2 ratio. When the working volume required is greater than 850 mL, the remaining volume of medium will be the volume in the recirculation bottle. When the working volume is less than 850 mL, recirculation will not be required. The minimum working volume in the iCELLis Nano bioreactor is 600 mL so that the cell culture medium can reach the top of the fixed-bed column to form a ‘falling film effect’ during agitation to provide gas exchange in the cell culture. If the volume in the bioreactor is less than 600 mL, cell culture medium circulation through the fixed-bed column would not be possible, resulting in no gas exchange. In this scenario, the V/S ratio needs to be increased to meet the minimum working volume of 600 mL.
| Surface area (cm2) | Media volume / surface area ratio (mL/cm2) | Total working volume (mL) | Volume in the recirculation bottle (mL) |
| 5300 | 0.2 | 1060 | 210 |
| 8000 | 0.2 | 1600 | 750 |
| 10600 | 0.2 | 2120 | 1270 |
| 16000 | 0.2 | 3200 | 2350 |
| 26500 | 0.2 | 5300 | 4450 |
| 40000 | 0.2 | 8000 | 7150 |
The second step is to determine the recirculation flow rate between the iCELLis bioreactor and the recirculation bottle. The aim is to ensure sufficient mixing to achieve homogeneity in cell culture media composition in both vessels. If the flow rate is too low, the nutrients are not well mixed between the iCELLis bioreactor and the recirculation bottle with the risk of nutrients depletion and accumulations of cell culture metabolites and inhibitors in the iCELLis bioreactor. If the flow rate is too high, room temperature media flowing from the recirculation bottle cools down the media in the iCELLis bioreactor. It then becomes challenging to maintain temperature at the setpoint (usually 37°C) in the iCELLis bioreactor. A flow rate above 13.5 mL/min in the iCELLis Nano Bioreactor would require additional equipment such as heating blanket or water bath to maintain the temperature set-point in the recirculation bottle. If the process is planned to be scaled-up in the iCELLis 500+ bioreactor, there are constraints that need to be considered during process development in the iCELLis Nano bioreactor. If the flow rate is greater than 1 L/min, a double jacket mixer tank is required to maintain the temperature in the bioreactor. Moreover, the maximum theoretical recirculation flow rate is 2.4 L/min if using the low flow manifold (¼ in. internal diameter (ID) 3/8 in. outer diameter (OD)) and 5.4 L/min if using the high flow manifold (3/8 in. ID ½ in. OD). Translation of the flow rate from the iCELLis Nano bioreactor to the iCELLis 500+ bioreactor is usually performed by using a factor of 125. This is because the iCELLis 500+ bioreactor with the same fixed-bed height and compaction has a surface area that is 125 times larger than its iCELLis Nano bioreactor counterpart.
Considering the above-mentioned factors, it is important to determine an optimal flow rate. A good starting point is to recirculate 1 or 2 working volumes per day and doubling the flow rate to match the doubling time of the cell culture. For example, a 4 m² iCELLis Nano bioreactor using a V/S ratio of 0.2 requires 8 L of total working volume. The proposed recirculation flow rate can be = 5.5 mL/min on the first day, 11 mL/min on the second day and 22 mL/min on the third day of cell growth. Alternatively, the maximum flow rate can be applied from the beginning of the process if the operator does not want to change it every 24 hours. In this case, the recirculation flow rate will be at a constant of 22 mL/min on all three days. As the flow rate is above 13.5 mL/min, the recirculation bottle may need to be placed in a water bath to be maintained at process temperature.
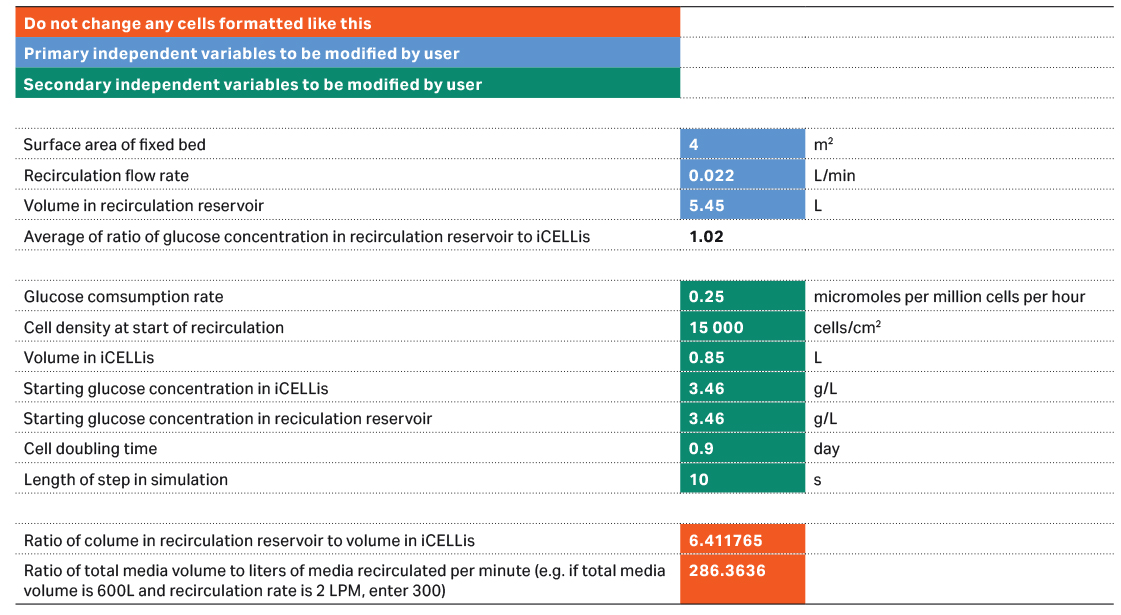
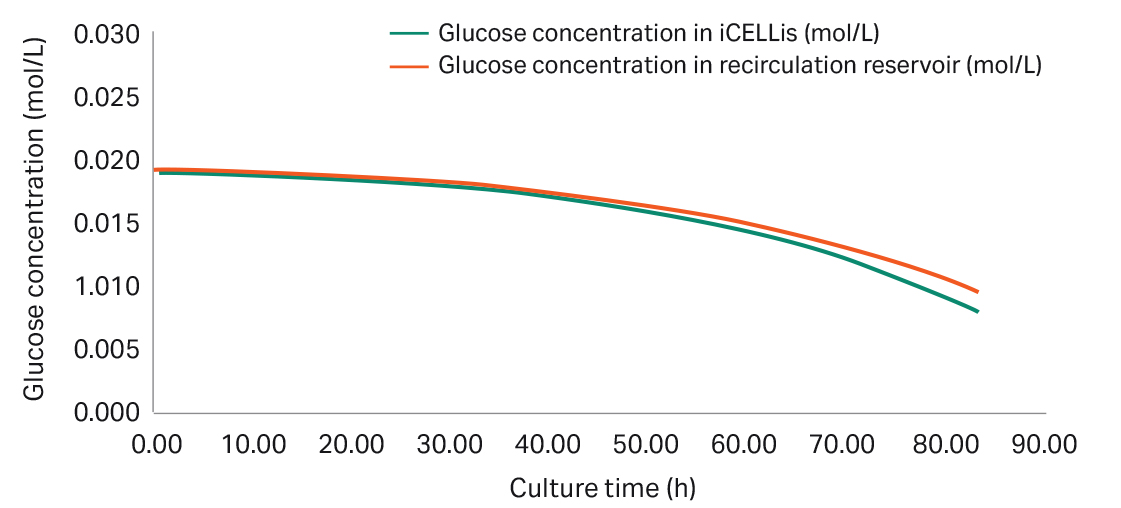
For customers who wish to develop and optimize their recirculation process, the flow rate can be variable and adjusted based on the glucose consumption rate of the cells. As cell density increases, the glucose consumption rate for the culture increases. Hence the optimal flow rate depends on the mass balance between cell doubling time and specific glucose consumption rate. To determine these two parameters, the experimental data needed includes cell count by carrier sampling, and glucose concentrations of media samples from both iCELLis Nano bioreactor and the recirculation bottle. Your Cytiva bioreactor applications specialist can provide an Excel template for this calculation (see Fig 3 and Fig 4). The recirculation flow rate should be adjusted such that the glucose concentration in the iCELLis bioreactor and in the recirculation reservoir is similar to each other (refer to Fig 4).
Fig 3. Mass balance tool to assess glucose consumption rate of the cells based on the cell growth and glucose concentrations.
Fig 4. Glucose concentration in iCELLis bioreactor and recirculation reservoir based on recirculation flow rate.
A last point to consider is the time to initiate recirculation. During inoculation and infection or transfection phase, recirculation is not recommended as it would result in the loss of cells, virus or transfection mix into the recirculation reservoir. In general, it is recommended to wait 24 hours after inoculation to help cell attachment if the cells secrete attachment factor. However, low cell culture nutrients or high metabolites and inhibitors may be important factors to initiate the recirculation loop earlier. Reactivation of the recirculation loop after the infection or transfection step is highly process dependent. Again, an optimal time needs to be determined to allow the virus or transfection mixture to enter the cells, while managing cell culture nutrients or high metabolites and inhibitors.
Considerations of perfusion
For the perfusion approach, customers will need to decide on the following parameters: flow rate, time to initiate, total duration, media composition and impact on downstream processing. In this section, we will explain the scientific rationale for different strategies and illustrate with examples from previously conducted studies.
Perfusion flow rate
One of the first considerations is to decide whether to perform perfusion at a fixed or variable rate. Thiscan be influenced by the purpose of performing perfusion. Fixed perfusion rate can be adopted toharvest viral vectors that are not stable in the bioreactor, e.g., lentivirus vectors, at an earlier time and topreserve the biological activity of the viral vectors. The flow rate can be fixed at a constant of 0.5 to 1working volume/day (1, 2). Conversely, variable perfusion rate can be performed to target the desired metabolite concentration such as glucose or lactate, in order to boost cell culture performance and/or increase virus titer, e.g., AAV/ lentivirus. The next step is to determine the optimal glucose concentration, and the target can be between 0.5 to 2 g/L of glucose in the bioreactor during initial process development. Valkama et al. (3) found that when targeting a lower glucose concentration of 0.5 g/L, the cells were able to proliferate well with less glucose. Overall, this led to a decrease in the use of the required perfusion medium (i.e. lower cost of goods (CoGs)) and total product volume for downstream processing. The optimal metabolite profile will depend on the cell line and product being produced.
When to initiate perfusion?
Another consideration is the time-point of the cell culture process when perfusion is initiated, either during cell growth phase or virus production phase. It is recommended to wait for the completion of the inoculation and infection or transfection phase before starting perfusion for the same reasons mentioned above – to prevent any loss of cells, virus or transfection mix. Firstly, small-scale experiments can be conducted to test if perfusion can improve cell growth in a shorter period of time. However, it is also important to determine if starting perfusion early will be cost-effective. Alternatively, perfusion can be initiated during the virus production phase. This may help to increase virus titer, as unstable viral vectors are harvested earlier, product aggregation is prevented or cell metabolism is improved. As an example, Valkama et al. (3) and Leinonen et al. (4) started perfusion on culture day 1, whereas Yoganathan et al. (1) and Pelletier et al. (2) initiated perfusion after transfection. It may also be helpful to develop analytical methods to measure the total functional virus particles produced, in addition to measuring the total virus particles produced, during various time-points of the cell culture process. This can be used to determine the optimal time to initiate perfusion.
If perfusion is initiated during the virus production phase, the perfusion duration is typically the entire virus production phase (~48 to 96 hours), wherein perfusion is started between 4 to 24 hours post- transfection (1, 2). As perfusion progresses, it is important to monitor cell viability as it will directly affect the product titer and downstream processing. A significant decrease in cell viability results in cell lysis and accumulations of DNA, RNA, and host cell proteins. This will require clarification filters with a larger surface area, thus increasing the CoGs. If cell viability was observed to drop significantly, the perfusion duration should be shortened.
Perfusion media
The composition of the perfusion media should also be considered. The supplementation of fetal bovine serum (FBS) in media may be critical to provide nutrients for efficient cell growth e.g., HEK293 cells. Moreover, FBS may also help to protect the stability of selected products such as lentivirus vectors (4).
However, there is a concern over the stability and lot-to-lot consistency of FBS. Furthermore, the presence of FBS in cell culture media will pose challenges in downstream processing, and there will be availability issue of FBS in the long run which will be a concern for business continuity. Therefore, manufacturers are now developing serum-free media. Another factor is the glucose concentration in perfusion media. High glucose in perfusion media may be required for certain types of cell growth, as this may benefit by reducing the total volume of medium, leading to smaller volumes for downstream processing (3). The concentration of glucose in perfusion media may need to be increased as the culture progresses, and this should be optimized during process development. Throughout the development of perfusion media formulation, it is important to evaluate product quality to check for any changes to cell metabolism.
Impact on downstream processing
Perfusion processes in the iCELLis bioreactors typically produce a cleaner product with fewer impurities such as host cell DNA and host cell proteins. This is due to cells being immobilized on carriers that allow the fixed bed to serve as a natural cell retention device. Therefore, for extracellular viruses, the biomass burden is much reduced for the clarification step and filters are less likely to be fouled. Perfusion processes usually result in higher product titer and productivity (5, 6). As a result, it is likely that a greater capacity on chromatography devices is required. This can help minimize any product loss and maximize product recovery and yield. During perfusion, as product is continuously being harvested, it creates the opportunity for downstream processing such as clarification and chromatography steps to be carried out in a continuous manner. A semi or continuous purification process can prevent degradation of unstable virus or aggregation of product as the waiting time before processing is reduced or eliminated. Combined with a continuous upstream process, this opens up the window for a continuous bioprocessing workflow.
Media exchange in the iCELLis 500+ bioreactor
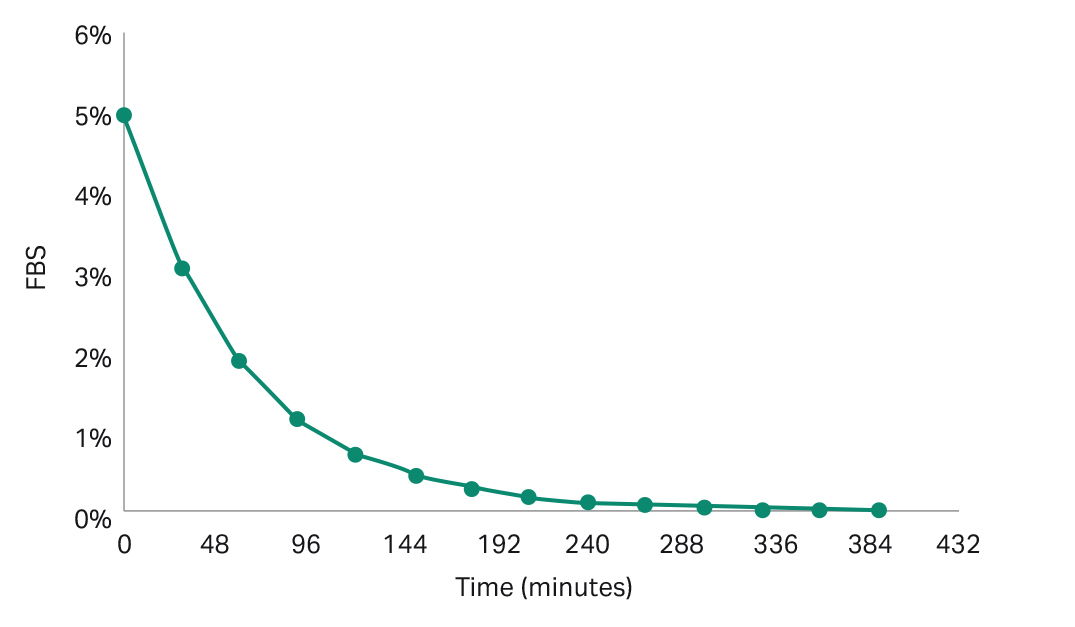
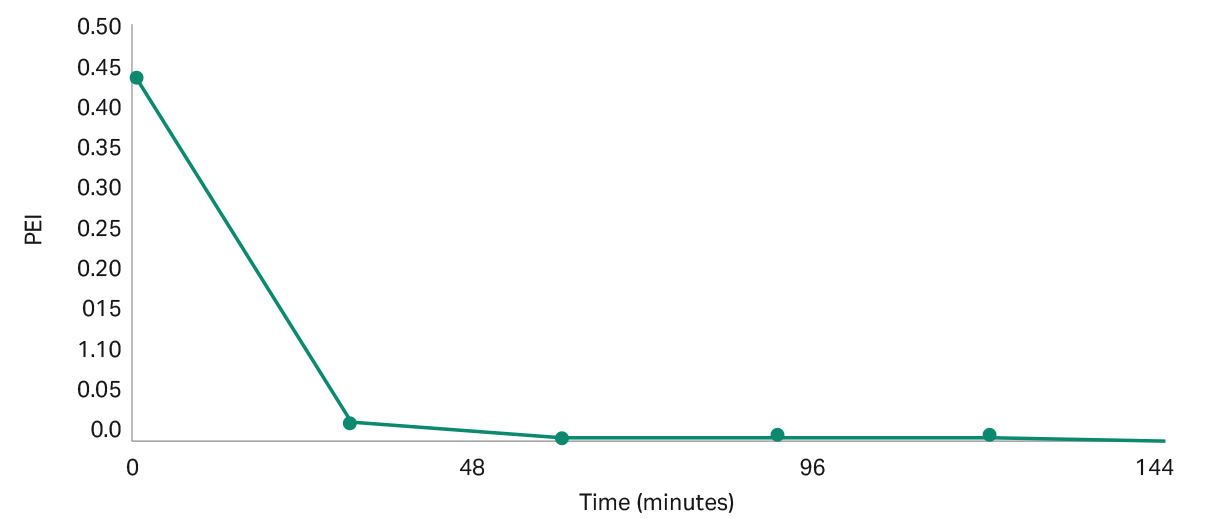
For the iCELLis 500+ bioreactor, media exchange to remove serum or polyethyleneimine (PEI) from the bioreactor will be challenging to perform due to the large volumes involved (60 to 80 L of working volume). An alternative solution is to perform fast perfusion into the bioreactor as a replacement for complete media exchange. For an initial serum concentration of 5%, and a dilution rate of 0.02 min -1, the serum concentration can be reduced to lower than 1% within 2 hours (Fig 5). For an initial PEI concentration of 43.7 mg/L and a dilution rate of 0.11 min -1, the PEI concentration can be reduced to 1.5 mg/L after 30 minutes (Fig 6). Your Cytiva bioreactor applications specialist can provide the Excel templates for the calculations.
Fig 5. Concentration of FBS over time, starting with an initial FBS of 5 % and dilution rate of 0.02 min -1.
Fig 6. Concentration of PEI over time, starting with an initial PEI of 43.7 mg/L and dilution rate of 0.11 min -1.
Summary and conclusions
The iCELLis fixed-bed bioreactor technology allows the spatial separation of the adhered cells in the fixed-bed, enabling easy implementation of strategies such as cell culture media recirculation, exchange, and perfusion or continuous processes. This presents an avenue to enhance productivity and quality improvement with a much smaller footprint than the traditional flatware process. Ensuring the development of an efficient scalable process, necessitates determining an appropriate process strategy at the process development stage i.e. at the bench scale iCELLis Nano bioreactor. The decision to implement and optimize recirculation and/or perfusion requires development to understand the cell growth, metabolism, media capacity, productivity, product stability, etc. at this scale. This optimized iCELLis Nano bioreactor process can then be scaled up to the manufacturing scale iCELLis 500+ bioreactor with minor improvisations, considering the scale of operations such as media volumes to handle, flow rate restrictions, and temperature control for media/perfusate. There is no unique solution that can work for every process, product, or platform and this document offers guidance to understand the benefits and challenges on the application of these strategies and simplies their evaluation for your process. Contact your Cytiva bioreactor applications specialist or sales representative for more information on the product.
- Yoganathan P, Lee AC, Hyatt N, et al. Perfusion Enables Increased Lentivirus Production using the iCELLis ® Bioreactor System. Cytiva, Ottawa Hospital Research Institute, 2020.
- Pelletier I, Pasupuleti V, Agnihotri P, Do Y, Sandalon Z, Bayne K, Scale up of a lentiviral production process from the iCELLis ® Nano bioreactor to the iCELLis 500+ bioreactor. Cell & Gene Therapy Insights, 2021;7(9);1023. doi:10.18609/cgti.2021.134
- Valkama AJ, Leinonen HM, Lipponen EM, et al. Optimization of lentiviral vector production for scale-up in fixed-bed bioreactor. Gene Therapy. 2018;25;39-46. doi:10.1038/gt.2017.91
- Leinonen HM, Lipponen EM, Valkama AJ, et al. Preclinical Proof-of-Concept, Analytical Development, and Commercial Scale Production of Lentiviral Vector in Adherent Cells. Molecular Therapy: Methods & Clinical Development. 2019;15;63-doi:10.1016/j.omtm.2019.08.006
- Mendes JP, Fernandes B, Pineda E, et al. AAV process intensification by perfusion bioreaction and integrated clarification. Frontiers in Bioengineering and Biotechnology. 2022;10;1-11. doi:10.3389/fbioe.2022.1020174
- Efficient Production of rAAV in a Perfusion Bioreactor Using an ELEVECTA ® Stable Producer Cell Line. Genetic Engineering & Biotechnology News, [Online]. Available: https://www.genengnews.com/sponsored/efficient-production-of-raav-in-a-perfusion-bioreactor-using-an-elevecta-stable-producer-cell-line/. [Accessed 27 Feb. 2023].