This article is inspired by a Tools and Technology presentation at American Society of Gene & Cell Therapy (ASGCT) 2021 annual conference.
Adeno-associated virus (AAV), a common gene therapy vector, is typically purified using chromatography resins developed for much smaller recombinant proteins. Processing time is usually long for affinity capture, and concentration by tangential flow filtration (TFF) is therefore oftenrequired. Fibro chromatography has an open structure of electrospun filters that allows for high flow rates and binding capacities. These properties make it very attractive for AAV capture. In this study we and a biomanufacturer evaluated Fibro prototypes for this purpose. We found that the fast residence times allowed much shorter process time with similar recovery and purification performance as affinity resins. Furthermore, the Fibro technology has the potential to eliminate the need for TFF and fits on existing chromatography systems.
AAV vectors for gene therapy
Viral vectors like AAV are one type of advanced medicinal therapeutic product (ATMP). Gene therapy is a rapidly expanding field with hundreds of clinical trials ongoing; AAV is one of the most important vectors used, especially for in vivo applications. There’s a lot of investment going on in this area, and many pharma companies are trying to bring these curative therapies to the market. Therefore, we’re in a situation where we need more capacity worldwide. But to make these new gene therapies available for very large patient populations and expand access around the globe, we need to intensify the processing (i.e., do process intensification) so that gene therapies can be manufactured at lower cost. Process intensification is about addressing the pain points in the processing workflow, some of which are shown in Figure 1.
The current state of AAV purification
Fig 1. Diagram showing key midstream and affinity capture steps in an AAV production workflow.
In downstream processing, AAV titers are relatively low. And this means that the affinity columns are usually over-dimensioned (i.e., larger than needed) to make sure that the large bioreactor volumes can be processed within a reasonable timeframe. Even with these larger columns, AAV affinity chromatography can still take up to 24 h. One option to shorten the time of this step is to concentrate the feed. But this concentration step can take 12 to 24 h, and it has a negative impact on the overall recovery.
Fibro technology addresses midstream and affinity pain points
Fibro technology, a cellulose-based material made from electrospun fibers, can help to address pain points around the concentration and capture steps. This technology allows high flow rates and high binding capacities, as shown by the Fibro PrismA units we launched in 2020 for mAb capture. The benefit for ATMP processing, which encompasses larger sized entities such as viral vectors and mRNA, is described in Figure 2.
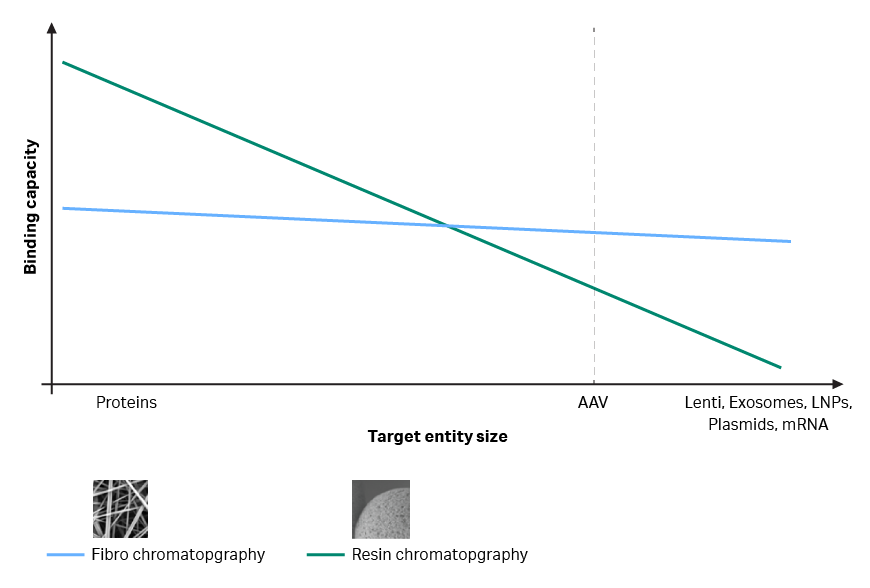
Fig 2. Graph showing how entity size impacts binding capacity of resin vs Fibro chromatography for AAV capture.
Binding capacity is on the y-axis, and size of the target entity is on the x-axis. Relatively small target entities like proteins are on the left, and entity size increases as you move along the x-axis to the right. The green line shows traditional resin chromatography. Resin technology is associated with a drop in capacity, that’s more pronounced the larger the target molecule is. This drop in capacity occurs because the large viruses and other biological entities can’t access all the ligands that are derivatized on the resin. Viruses and other large molecules can’t get into all the small pores.
Fibro chromatography uses a matrix with a much more open structure. And this means that the majority of derivatized ligands can bind a large molecule. For large entities such as viral vectors, we will have higher capacity on Fibro than we have on a resin chromatography column. This, in combination with the benefit of the open structure, means that the binding doesn’t depend on diffusion. So, no time is needed for the viruses to diffuse into a chromatography bead. And this means that the residence times on Fibro are in seconds rather than in minutes.
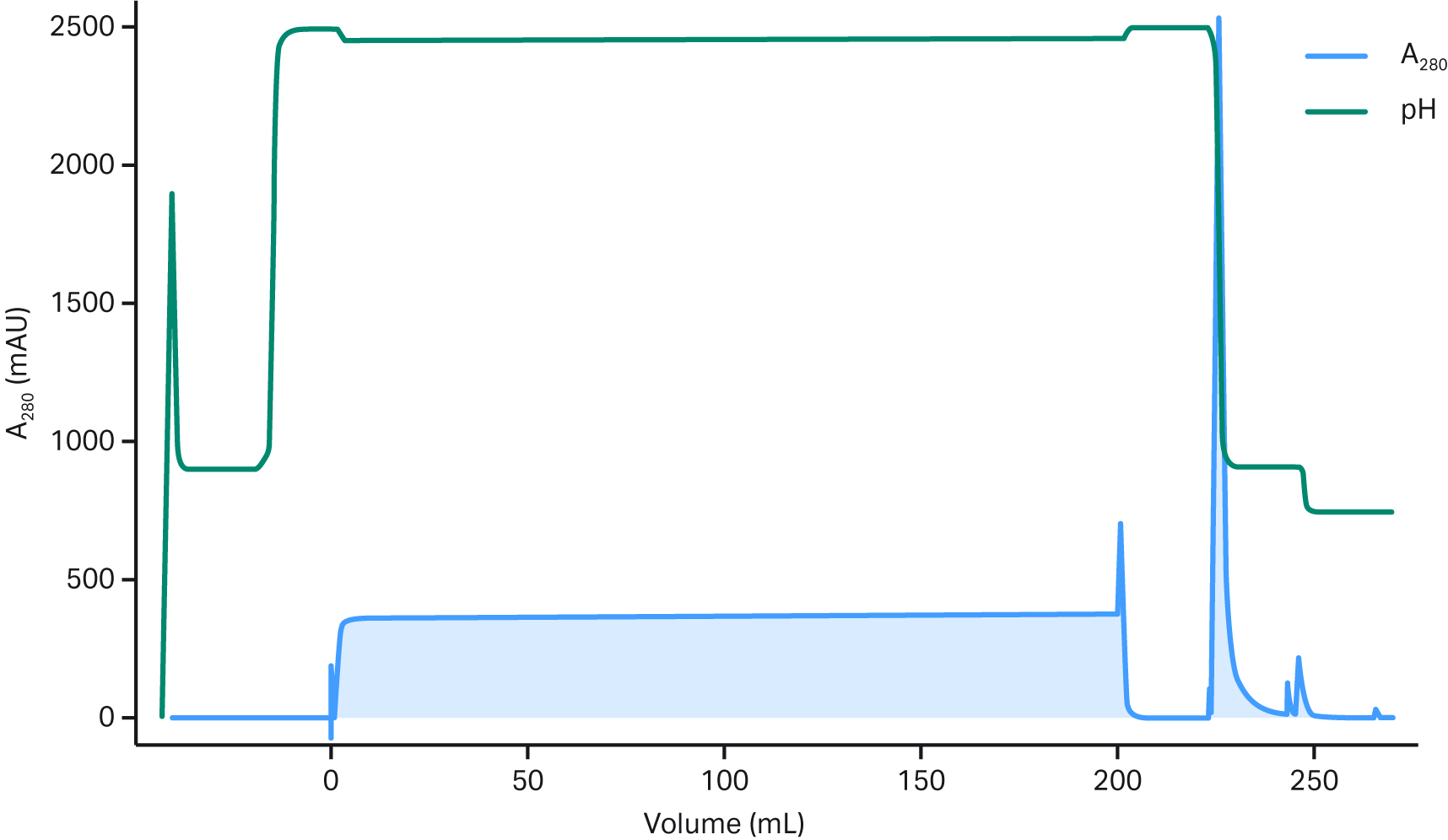
Figure 3 shows an example of AAV5 capture using a Fibro prototype unit.
Fig 3. Example chromatogram from an AAV5 capture step using a 0.4 mL Fibro prototype derivatized with an AAV binder. NFF is normal flow filtration. TFF is tangential flow filtration.
Based on our results, the capacity of the 0.4 mL prototype derivatized with an AAV binder is typically 1–4 × 1014 capsids/mL. The residence times used in these evaluations are in the range of one to five seconds. Looking at Figure 3, even though we're running these high flows, we have an even pressure during the loading phase, which in this example took about 20 min. Our results show that you can capture up to 1 L harvest feed with the 0.4 mL Fibro unit in 1 to 2 hours depending on harvest feed. The impurity reduction (99% for both host cell protein and DNA) and recovery (60% to 90%) are similar to what we're used to seeing from AAV capture with chromatography resins.
Biomanufacturer case study for AAV5 purification
The midstream approach for Fibro is similar to the approach for resins. When we test a new feed, we typically start with normal flow filtration (NFF). If the pressure increases during loading, you can add an uncharged depth filtration step. For really challenging feeds you need to add a charged depth filter or even go into more advanced clarification including, for example, precipitation in combination with the depth filter and a normal filter.
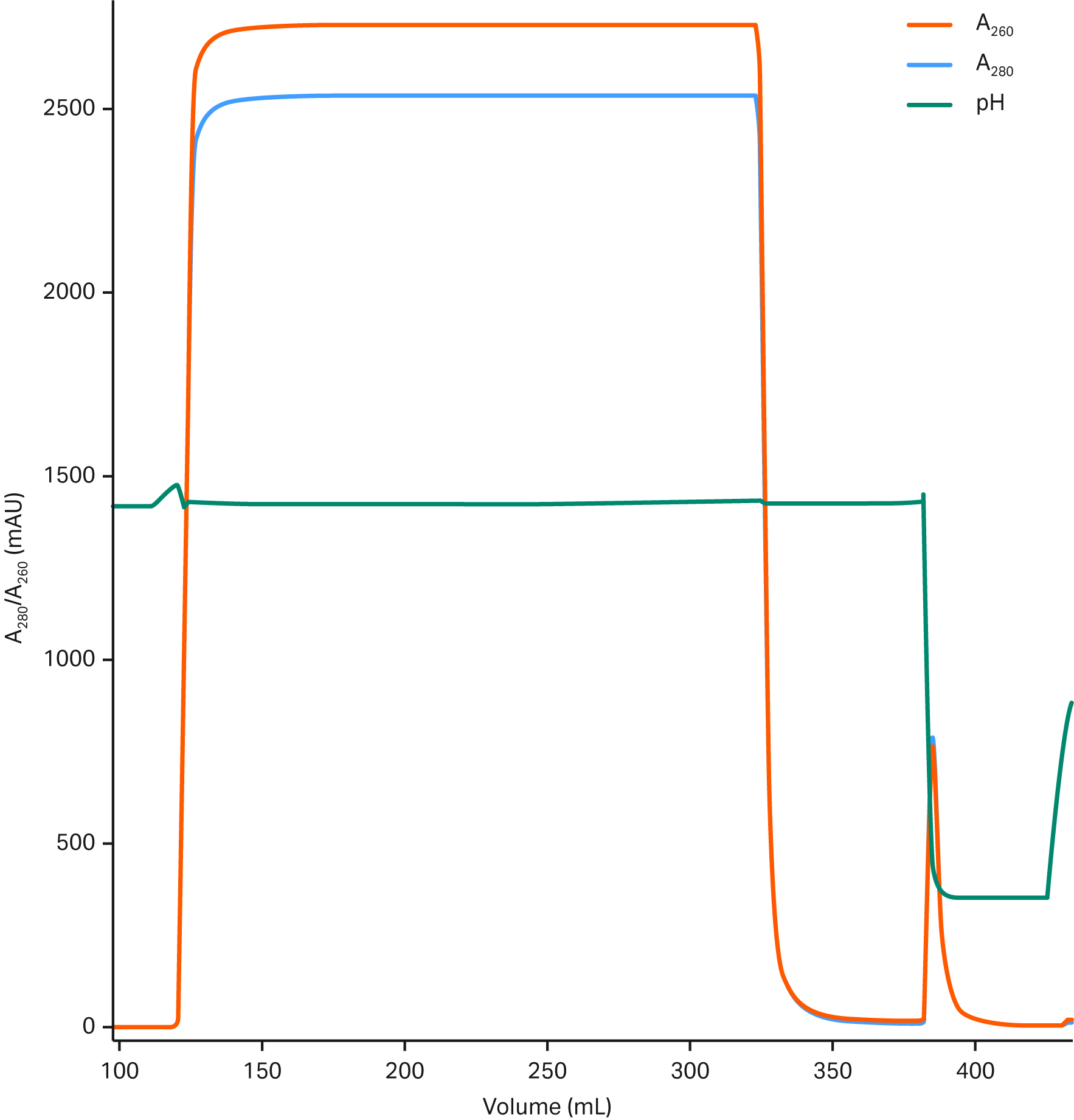
To make sure that we can apply this same clarification approach to feed streams besides our internal ones, we’ve asked collaborators to evaluate our Fibro prototypes. Figures 4 and 5 show data from one collaborator, Professor Du Zengmin at Belief Biomed in China.
Fig 4. Clarified AAV5 sample subjected to affinity capture on a 0.4 mL Fibro prototype derivatized with an AAV binder. Data courtesy of Belief Biomed.
For the chromatogram in Figure 4, the AAV5 feed was clarified with an uncharged depth filter followed by a 0.2 µm normal flow filter. With this clarification method the residence times were in seconds and the loading time was reduced substantially compared with an affinity resins. The recovery was > 80% with purification performance similar to corresponding resins. There is limited flow through, even though we're loading at 2.4 s residence time, which means that these 400 µL prototypes were run at around 10 mL/min.
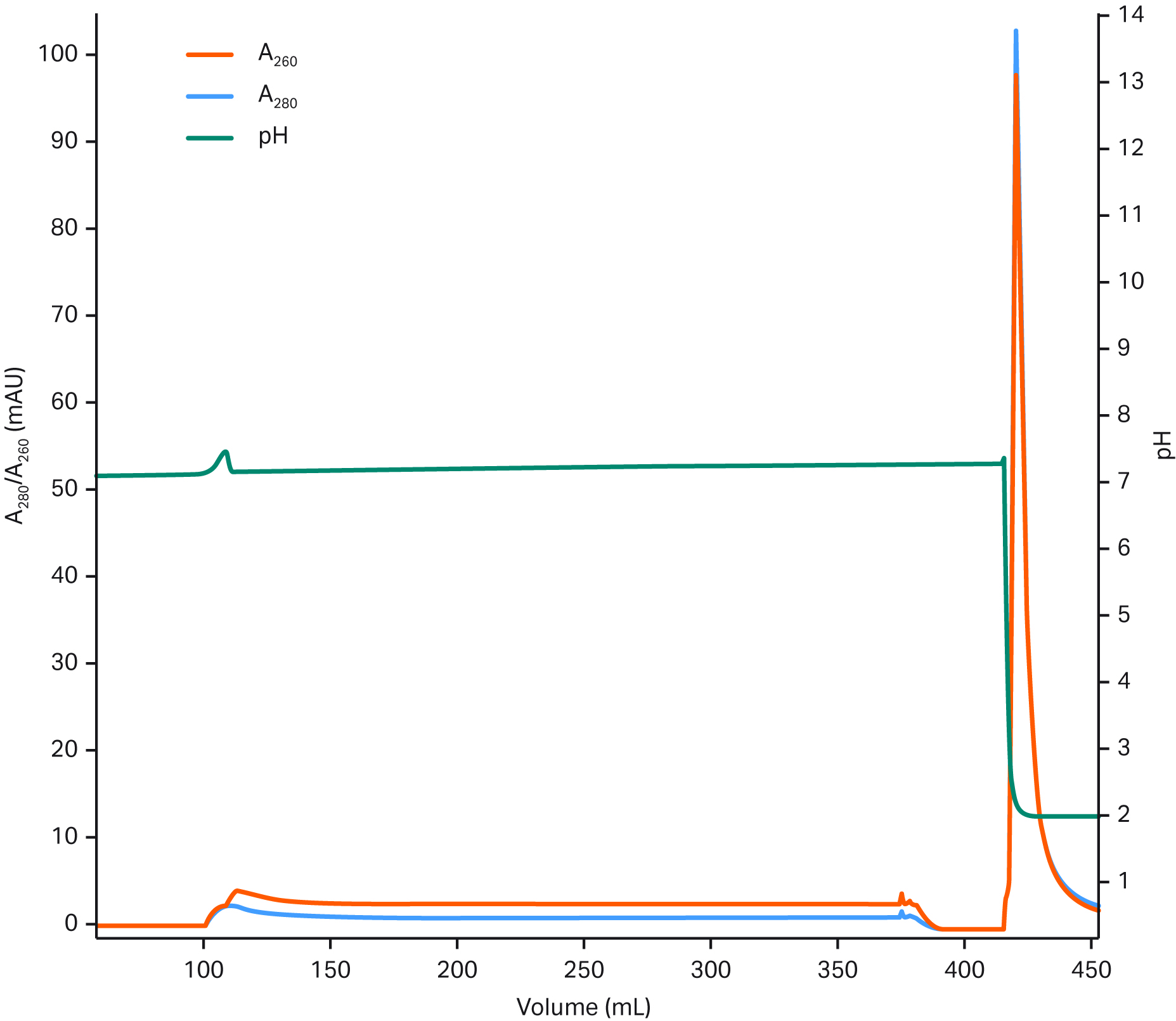
Figure 5 shows a chromatogram from capture of a prepurified AAV5 sample using a Fibro prototype.
Fig 5. Prepurified AAV5 sample subjected to affinity capture on a 0.4 mL Fibro prototype derivatized with an AAV binder. Data courtesy of Belief Biomed.
The pressure curve for this prepurified sample was similar to the curve for the clarified sample, which shows promise for scaling up scenarios. Also, if titers are lower, the sample volumes will exceed the 500 volumes shown previously. In Figure 5, 625 Fibro volumes were loaded, though this was still underloaded relative to the maximum capacity of the prototype.
Fibro allows for high capacity at short residence times
Figure 6 illustrates the difference between fiber chromatography and resin chromatography for AAV capture.
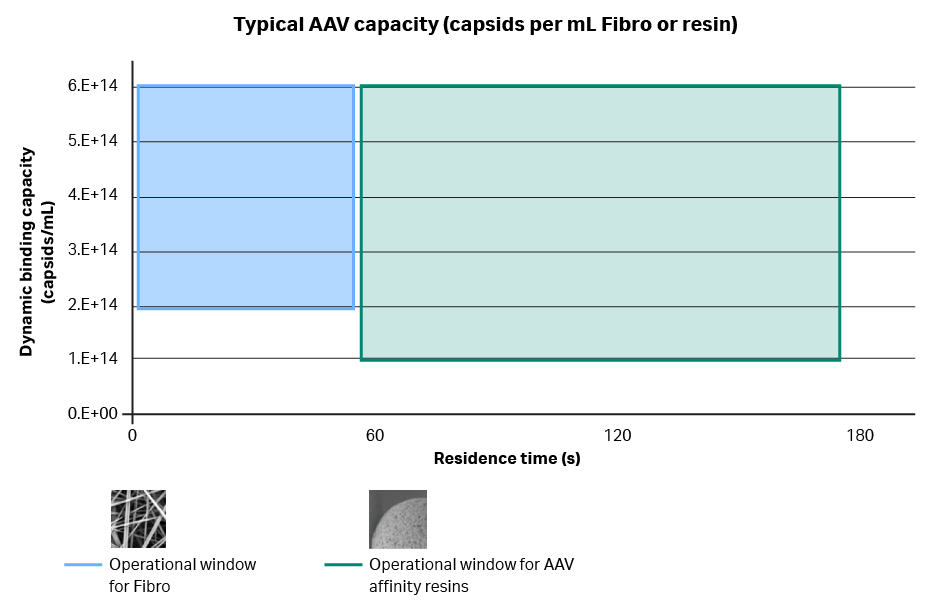
Fig 6. Graph of dynamic binding capacity vs residence time for Fibro and AAV affinity resins.
Dynamic binding capacity is on the y-axis, and residence time is on the x-axis. With Fibro for AAV capture, residence times are less than a minute. But for resin chromatography, residence times under one minute aren’t common. Despite the lower residence time, our experiments show that Fibro has about double the capacity to resins.
In summary, we see that Fibro chromatography allow for substantially shorter processing time for the affinity capture step. Also, the quick loading time means that you have an opportunity to remove the TFF concentration. In our experience, removing the TFF doesn't require any major changes to your clarification method. And the performance of the AAV capture on fiber is similar regarding impurity reduction and recovery as seen with similar resins. Finally, Fibro is a prepacked unit that’s simple to set up and fits existing instrumentation equipment, as shown in Figure 7.

Fig 7. Prepacked Fibro units fit easily on the same ÄKTA chromatography systems that you use for resin chromatography.
Acknowledgements
We kindly acknowledge Professor Du Zengmin from Belief Biomed for evaluating our prototypes and granting us permission to publish his data.
Learn more about Fibro and other solutions for AAV production and purification.