Overview: Reducing CO2 emissions doesn’t just help the planet — it also helps drive efficiency in supply chains, increasing the profitability and sustainability of business models. It can also help remove redundancy in cell therapy shipping and manufacturing processes, and because of this, there’s now concerted effort to reduce emissions in the cell therapy supply chain.
One of the most carbon-intensive aspects of cell therapy manufacturing is cold-chain logistics. We have previously shown that switching to a liquid nitrogen-free (LN2-free) cryopreservation process can reduce CO2 emissions by 87%, and cooling costs by > 95%. In this application note, we consider shipments within the cell therapy workflow, from initial patient sample to final cell therapy arrival at the patient’s side.
We determined that by streamlining the delivery processes with efficiency-led design and smart logistics, manufacturers can reduce the financial costs of transport and CO2 burden by 57%. This is made possible by the VIA Capsule™ shipper’s smart disinfection design, which reduces the number of required shipments for any given cell therapy manufacturing process.
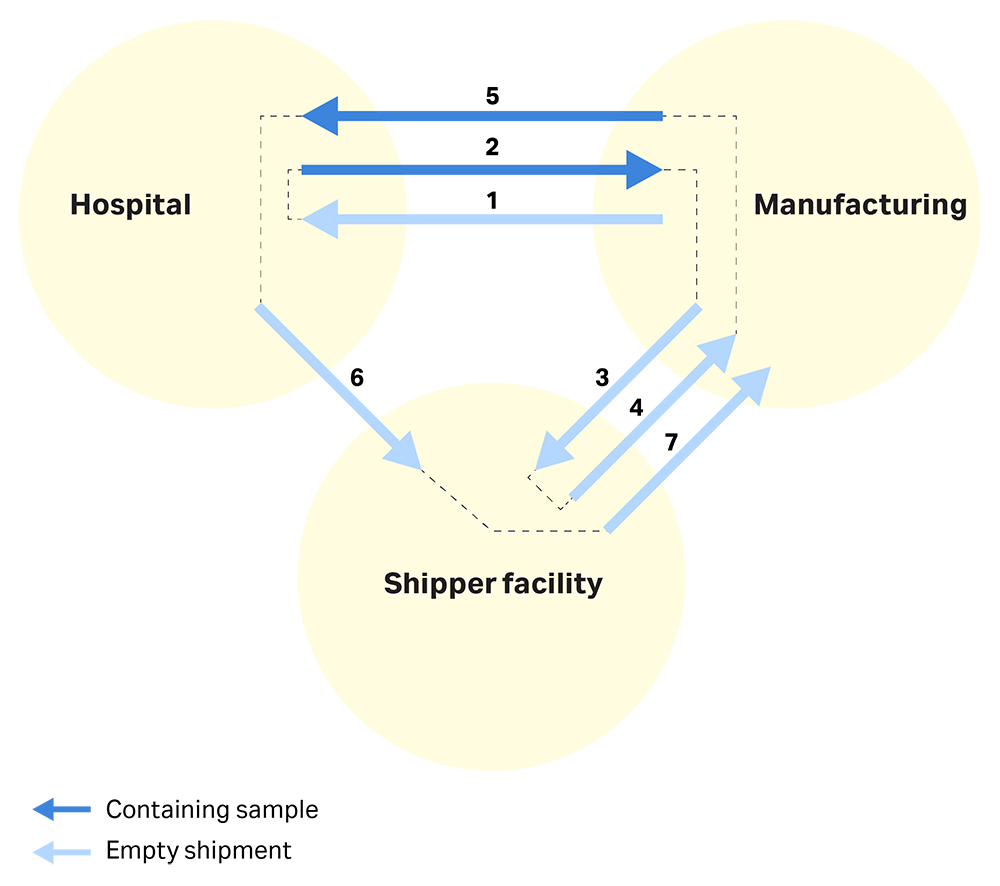
The current shipping model: The shipping process is a substantial cost and logistic burden in the overall cell therapy manufacturing workflow. Manufacturers most commonly use dry shippers. We describe a typical shipping process below and in Figure 1.
- The shipping vessel moves from the manufacturer’s warehouse to the hospital.
- A patient sample is collected and shipped to the centralized manufacturing facility.
- After transporting a biological sample, the vessel is sent to a shipper facility to be disinfected.
- The disinfected vessel returns to the manufacturing facility.
- The final cell therapy ships from the manufacturing facility to the patient’s hospital.
- The shipping vessel is sent for disinfection.
- The shipping vessel returns to the manufacturing facility until needed, or to the hospital to collect and transport another patient sample.
Fig 1. A typical shipping process; the three key locations are the manufacturing site, the shipper facility where disinfection and LN2 filling take place, and the hospital where the patient is located.
Exact process steps can vary. But a common step in all processes is disinfecting the LN2 shipper, which occurs several times throughout the process. Typically, manufacturers disinfect a LN2 shipper and fill it with liquid nitrogen at an outsourced specialist courier before shipping it to the clinic or the manufacturing site. Then they’ll return it empty to the specialized courier between shipments for additional disinfection steps, and to add new LN2 coolant. Usually only the specialist courier can refill the LN2 shipper, which requires additional, and costly, empty shipments.
The cost of shipment, both financially and in terms of carbon footprint, directly relates to the number of movements the vessel makes — the weight of the cell therapy itself is minimal compared to the weight of the vessel and vehicle transporting it. This means that the carbon emissions of transporting a vessel — whether for disinfection, topping up with coolant, or moving a therapy — will be the same. The financial burden is similar too — while the shipment might be less critical when not containing a therapy, each day a shipper is out-of-action for cleaning or adding coolant makes it unavailable for biological use.
VIA Capsule™ system aims to eliminate both these inefficiencies with a smart disinfection design and by using electricity instead of LN2 for instrument cooling.
VIA Capsule™ system, shown in Figure 2, is a smart shipping system that uses a Stirling engine to maintain a low temperature. This electric-only power source removes the need for a liquid coolant. The system can maintain ultralow temperatures for up to five days, and you can plug it into a regular power supply at any point to extend this autonomy. It has a sleek design with no hard-to-reach areas or molecular sieves — you can clean it simply and easily on-site as opposed to at a specialized molecular sieve disinfection facility. This increases efficiency and reduces redundant shipments in your process.
Fig 2. The internal design of VIA Capsule™ system allows for rapid disinfection, with a single lid to enable recharging with electricity.
Why sterilization is required: Disinfection is essential for patient safety, and manufacturers apply it between each use of a shipping vessel. Sources of contamination include:
- LN2 itself, which can carry and disperse bacteria and fungus. This is why scientists exclude it from clean rooms.1,2
- Potential leaks from a biological sample that was incorrectly packaged or damaged.3,4,5
- Particles from the air that can enter whenever the lid is removed for access.
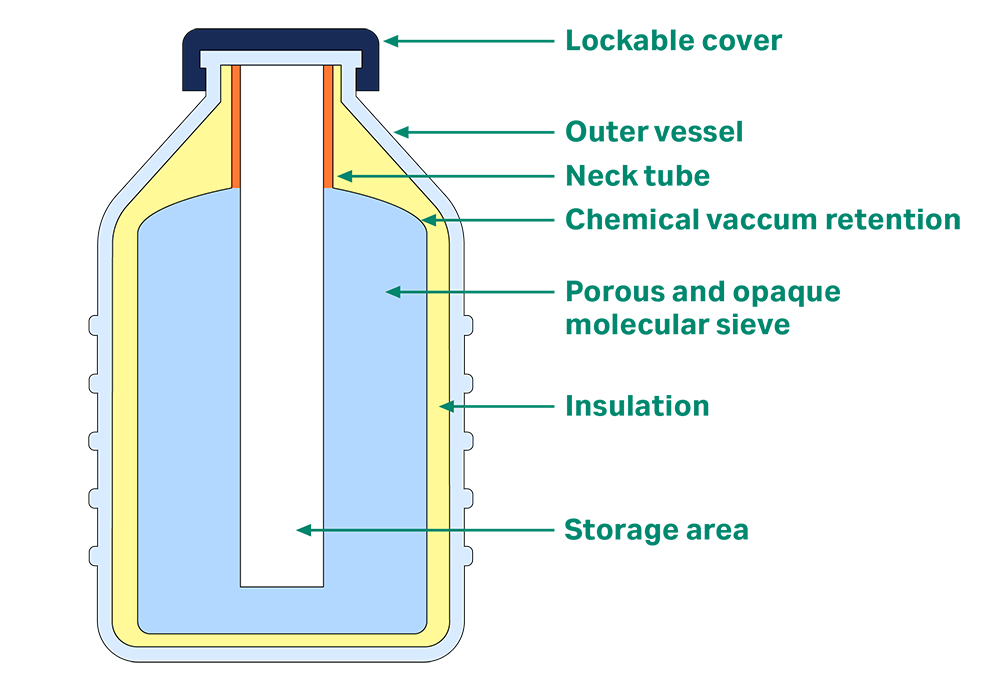
LN2 disinfection: Traditional LN2 shipping vessels present a unique challenge for disinfection. Not only does the act of adding LN2 to a shipping device introduce a large bioburden before a sample is even loaded1,2, but disinfecting the system also adds to the logistics and complexity of the operation. As LN2 cannot be used during shipping in closed spaces, which includes most road vehicles and aircraft, a conventional LN2 shipper (shown in Figure 3) has a special molecular sieve surrounding the sample container. The sieve absorbs the LN2 in a process known as charging. LN2 can no longer spill when it’s absorbed even if the vessel is tilted, making it suitable for shipment.
However, cleaning a molecular sieve is a significant challenge. The (nonsterile) nitrogen is in the sieve matrix, so manufacturers need to disinfect both the outside and the inside surfaces of the molecular sieve. Cleaning only the outer surface of the molecular sieve does not disinfect the interior surface.
Because manufacturers need to disinfect and refill LN2 shippers after each shipment, they must return the empty dry shipper to a specialist manufacturing site, warm it up, and perform a deep clean before adding new LN2. The special properties of the molecular sieve that make it useful for shipping are a severe handicap when it comes to disinfection. The sieve stops LN2 from leaking out, but it also hinders access when it comes to applying cleaning products. Even if you use UV light to disinfect an LN2 shipper, the light will not penetrate the molecular sieve, making it only partially effective.
Additionally, a specialist courier usually needs to charge the shipper, which makes the vessel usable for a limited period before it needs to be refilled again. For both practical and traceability reasons, topping up is usually not possible in clinical and manufacturing sites.
Fig 3. Elements of LN2 shipper. LN2 is poured into the system, which is then absorbed into the molecular sieve around the storage chamber.
Disinfection in the VIA Capsule™ system: The innovative design of VIA Capsule™ system, which uses a thermal mass and not a liquid coolant to cool, allows for full accessibility of each internal area of the instrument. This means users can easily disinfect the VIA Capsule™ system in a small amount of time using standard lab chemicals.
VIA Capsule™ shipper is composed of materials compatible with most standard laboratory cleaning products. Scientists usually disinfect the shipper with the following products:
- Spor-Klenz™ (hydrogen peroxide 1% and peroxyacetic Acid 0.08%)
- Klercide™ 70/30 denatured ethanol (70% ethanol)
- 70% isopropyl alcohol (70% IPA)
- Klerwipe™ (0.92% sodium chlorite and 0.29% didecyldimathylammonium chloride in deionized water, by volume, wipes)
- Purified water
The VIA Capsule™ shipper uses a disinfection design without molecular sieves, so you can access and disinfect every part of the sample chamber. This means you don’t need to ship the vessel to a disinfection or specialized courier site for cleaning. Additionally, there’s no need to top-up the liquid coolant — you only need to plug VIA Capsule™ shipper into a regular electricity supply to recharge for the next shipment, or you can use it as temporary storage until the therapy or biological sample is required.
These innovative features reduce the number of shipments, saving time and logistics efforts, and reducing the carbon footprint of the overall cold-chain delivery process.
Estimating logistics simplification: By using smart logistics, VIA Capsule™ system eliminates redundant shipments and substantially simplifies logistics.
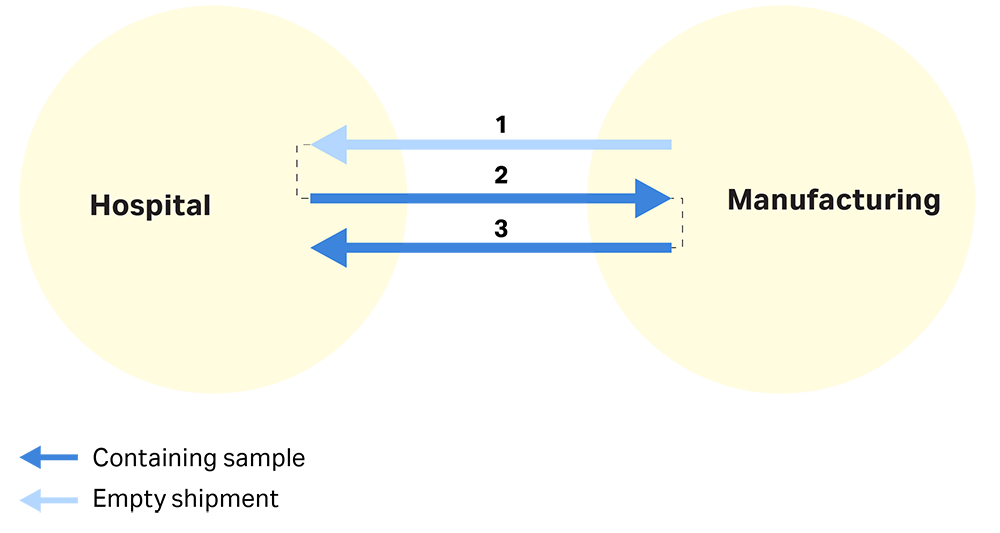
Fig 4. A schematic of a smart shipping process, where the shipping vessel no longer needs to be returned to the shipper facility for cleaning and LN2 refill.
This smart logistic chain can consolidate seven steps into three (see Figure 4 above):
- VIA Capsule™ system is delivered to the hospital.
- A patient sample is taken and shipped to a centralized manufacturing facility. VIA Capsule™ system is disinfected at the manufacturing site.
- The final cell therapy is shipped from the manufacturing site to the hospital. VIA Capsule™ shipper can then be used to deliver a new patient’s sample to the manufacturing site or returned when no longer required.
The physical shipping of a product is the largest contributor to CO2 emissions in the cold chain delivery process. A 100-mile journey in a van emits approximately 25 kg of CO2, compared with only 1 or 3 kg of CO2 that’s required to cool and maintain the low temperature of the of a LN2 dry shipper or a VIA Capsule™ shipper, respectively.6 Reducing the number of trips from seven to three reduces the carbon footprint of this process by 57% — 3/7 of the original.
To further reduce emissions, you can use the initial shipment for another product being manufactured concurrently. This reduces empty shipments and CO2 emissions by another third.
The above typical workflows are examples and can be tailored to individual products, as there can be multiple manufacturing and clinical sites within a cell therapy’s journey. Sometimes shipping is also required within a hospital, for example, between clinic and clean rooms. VIA Capsule™ system can support this and provide additional temporary storage as needed, eliminating the need for costly and carbon-intensive LN2-based transport and storage. This reduction in shipments also gives a two-fold improvement in capital costs; reducing extra shipments and letting each shipping instrument be used more effectively, reducing the total number of vessels required and the initial capital cost.
Summary and conclusions
Dry shippers must be transported empty many times in a typical delivery process, and the innovative design of smart shipping systems can enhance the current supply chain. Using VIA Capsule™ system can reduce the carbon footprint of cold-chain shipment by 57%, helping to reduce operating and capital costs. Importantly, reducing the number of steps in the cold chain gets therapies to patients faster, creating a meaningful impact in a more environmentally sustainable manner.
Literature references:
- Grout B.W.W., Morris G., 2009. Contaminated liquid nitrogen vapour as a risk factor in pathogen transfer. Theriogenology. 71(7):1079-82.
- Morris G.J., 2005. The origin, ultrastructure, and microbiology of the sediment accumulating in liquid nitrogen storage vessels. Cryobiology, 50(3):231-8.
- Tedder R., Zuckerman M., Brink N., Goldstone A., Fielding A., Blair S., et al., 1995. Hepatitis B transmission from contaminated cryopreservation tank. The Lancet, 346(8968):137-40.
- Fountain D., Ralston M., Higgins N., Gorlin J., Uhl L., Wheeler C., et al., 1997. Liquid nitrogen freezers: a potential source of microbial contamination of hematopoietic stem cell components. Transfusion, 37(6):585-91.
- Khuu, H., Cowley, H., David-Ocampo, V., Carter, C., Kasten-Sportes, C., Wayne, A., Solomon, S., Bishop, M., Childs, R. and Read, E., 2002. Catastrophic failures of freezing bags for cellular therapy products: description, cause, and consequences. Cytotherapy, 4(6), pp.539-549.
- Parmegiani, L., Accorsi, A., Cognigni, G., Bernardi, S., Troilo, E. and Filicori, M., 2010. Sterilization of liquid nitrogen with ultraviolet irradiation for safe vitrification of human oocytes or embryos. Fertility and Sterility, 94(4), pp.1525-1528.
- European Environment Agency. 2020. Average CO2 emissions from new cars and new vans increased again in 2019. [online] [Accessed 5 November 2021]