Summary: IL-2 stability study
Interleukin-2 (IL-2) is an essential reagent when manufacturing immune-based therapies, including T cells and natural killer (NK) cells. Like all reagents in cell manufacturing, IL-2 stability is key. In this study, we evaluated the stability of Cytiva’s Xuri™ IL-2 growth factor as a function of time, temperature, medium, IL-2 lot, and initial concentration. ELISA data over 8 days showed excellent stability over time (half-life of > 8 days).
The work described below was done with funding from the Federal Economic Development Agency (FedDev) at the Centre For Advanced Therapeutic Cell Technologies (CATCT) located at CCRM, Toronto, Ontario, Canada. Xuri™ IL-2 growth factor is manufactured under a certified GMP license and supports USP <1043> compliance.
Introduction: What is IL-2’s role in immunotherapy?
Interleukins are complex signaling cytokines produced by leukocytes to regulate immune response. IL-2 specifically augments NK-cell activity1 and influences T-cell differentiation into defined effector and memory populations to maintain immune homeostasis.2 IL-2 can be found in either soluble or matrix-bound form; however, soluble IL-2 is required for ex vivo T- and NK-cell expansion.
With growing momentum in the field of adoptive immune therapy, there is an increased demand for stable and reliable sources of IL-2 in cell therapy manufacturing. The stability of Xuri™ IL-2, a GMP-compliant product, was tested over 8 days in various media (serum-supplemented and serum-free) at different temperatures and starting concentrations. Overall, Xuri™ IL-2 was found to be stable during this period.
Materials and methods: Testing Xuri™ IL-2 stability
To verify that frozen samples could be used throughout the stability study, we started by comparing fresh IL-2-containing medium (samples run immediately on an IL-2 ELISA) vs. frozen samples run after 24 hrs at -80°C in complete Mini EDTA Free Protease inhibitors (Fig 1). This study confirmed that the frozen samples were representative of the fresh condition, so frozen samples were used for the remainder of the study. As such, IL-2 time course samples used in subsequent stability studies (Figs 2-4) were stored in complete Mini EDTA Free Protease Inhibitors (Sigma-Aldrich) at -80°C after incubation at 37°C for up to 8 days. Media tested included Xuri™ expansion medium supplemented with 5% human AB serum and AIM V™ Serum-Free Medium (Thermo Fisher Scientific).
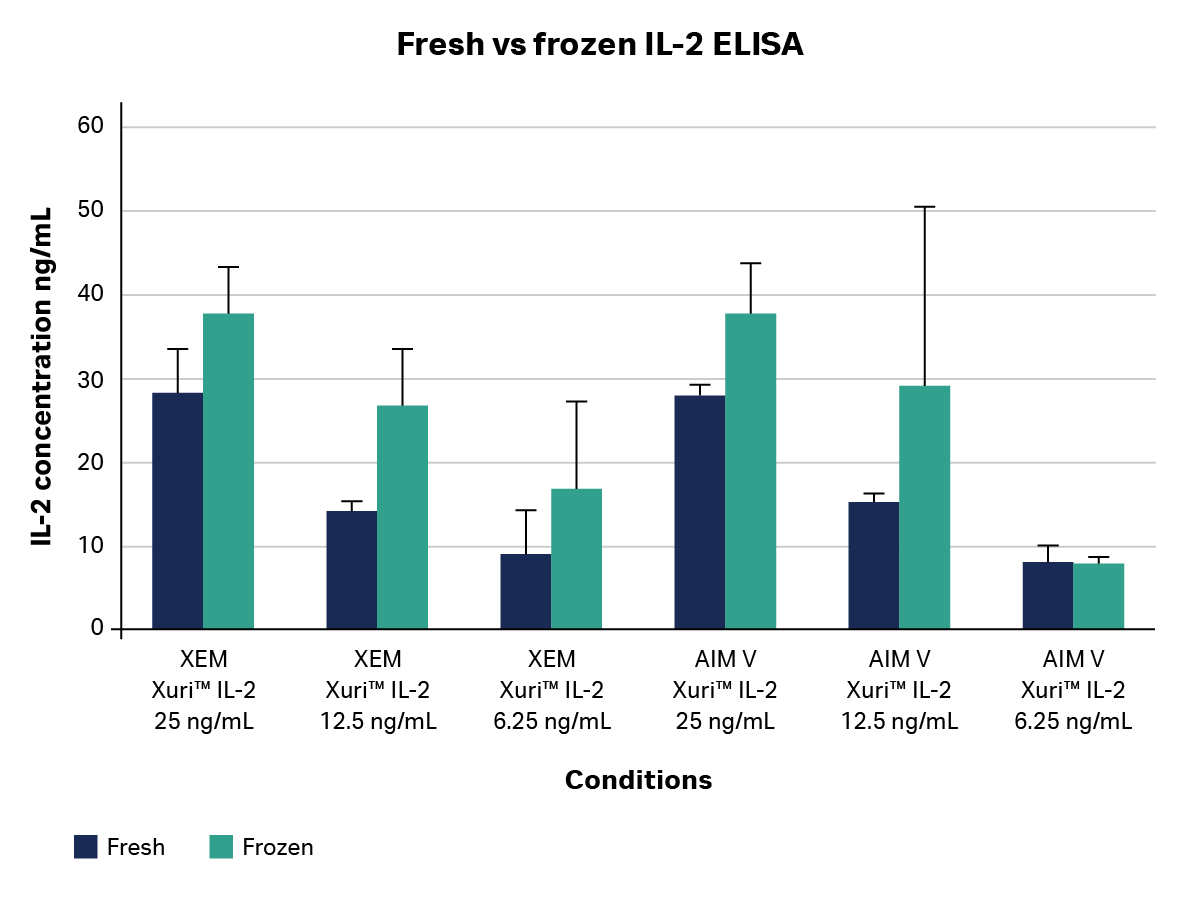
Fig 1. IL-2 concentration in fresh and frozen samples. GMP-compliant Xuri™ IL-2 was added to two different media: Xuri™ Expansion Media (XEM), containing 5% human AB serum, and AIM V™ Serum-Free Medium, at three different concentrations. Media were collected, protease inhibitors added, and samples run either immediately in an OptEIA™ IL-2 ELISA or frozen at -80°C for 24 h before being run in an OptEIA™ IL-2 ELISA to determine IL-2 concentration in each sample. Results are plotted as average of technical replicates (n=3) ± SD.
We tested two lots of GMP-compliant Xuri™ IL-2 to determine its stability. Stability studies (Figs 2–4) looked at the concentration of IL-2 in media as a function of time over an 8-day period. IL-2 was titrated and inoculated at a starting concentration of 25 and 12.5 ng/mL in two media formulations. Samples were collected on days 0, 1, 2, 3, 5, and 8 post-inoculation and measured using an OptEIA™ IL-2 ELISA kit (BD Biosciences). Concentrations were plotted as average ± SD and normalized to day 0 [relative units].
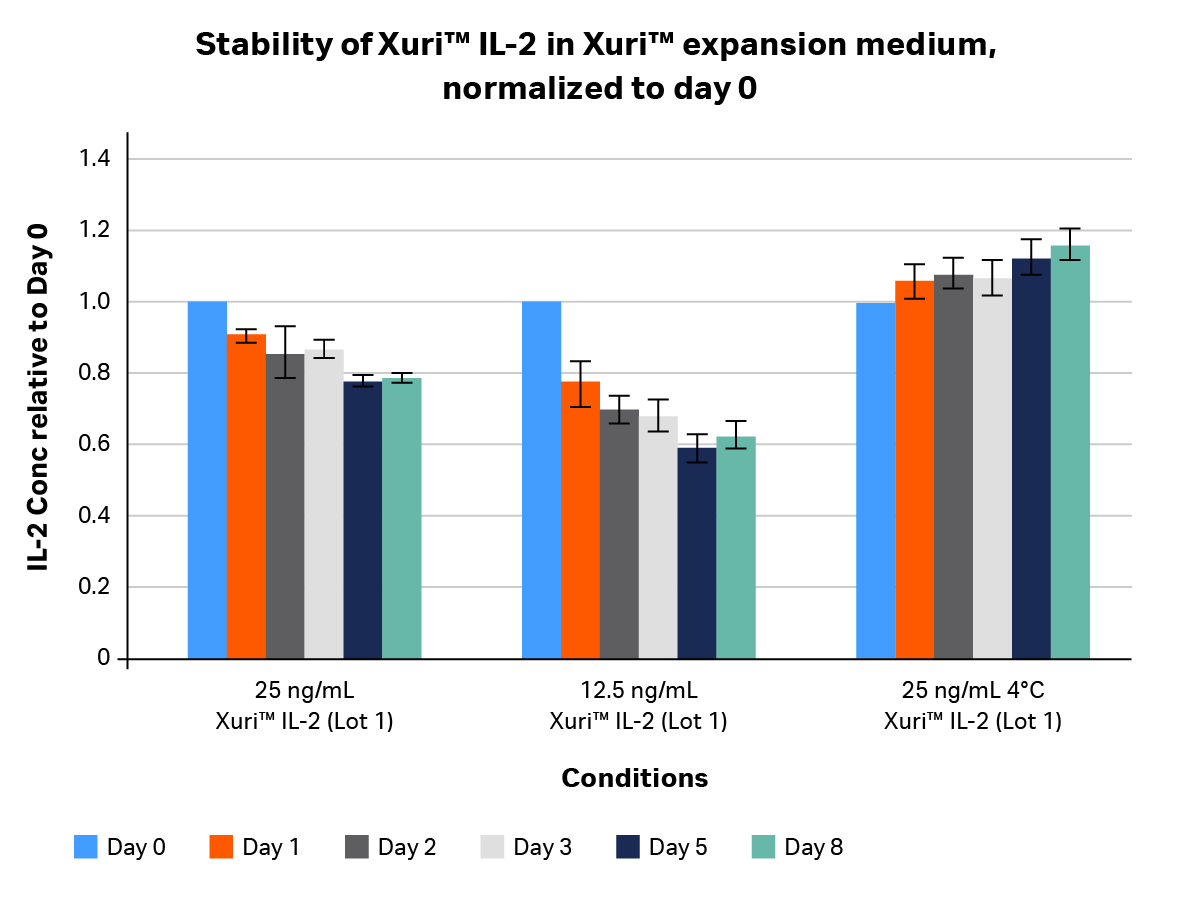
Fig 2. Stability profile of GMP-compliant Xuri™ IL-2 in Xuri™ expansion medium over 8 days, normalized to day 0. Xuri™ IL-2 at two different concentrations (25 and 12.5 mg/mL)was added to Xuri™ media containing 5% human AB serum and kept in a humidified tissue culture incubator at 5% CO2 and 37°C. A control sample of Xuri™ expansion medium with 5% human AB serum-containing Xuri™ IL-2 was left at 4°C. IL-2-containining media were collected at days 0, 1, 2, 3, 5, and 8, protease inhibitors added, and samples frozen in a -80°C freezer. After media from all time points were collected, frozen samples were thawed and IL-2 concentration was determined using an OptEIA™ IL-2 ELISA kit.
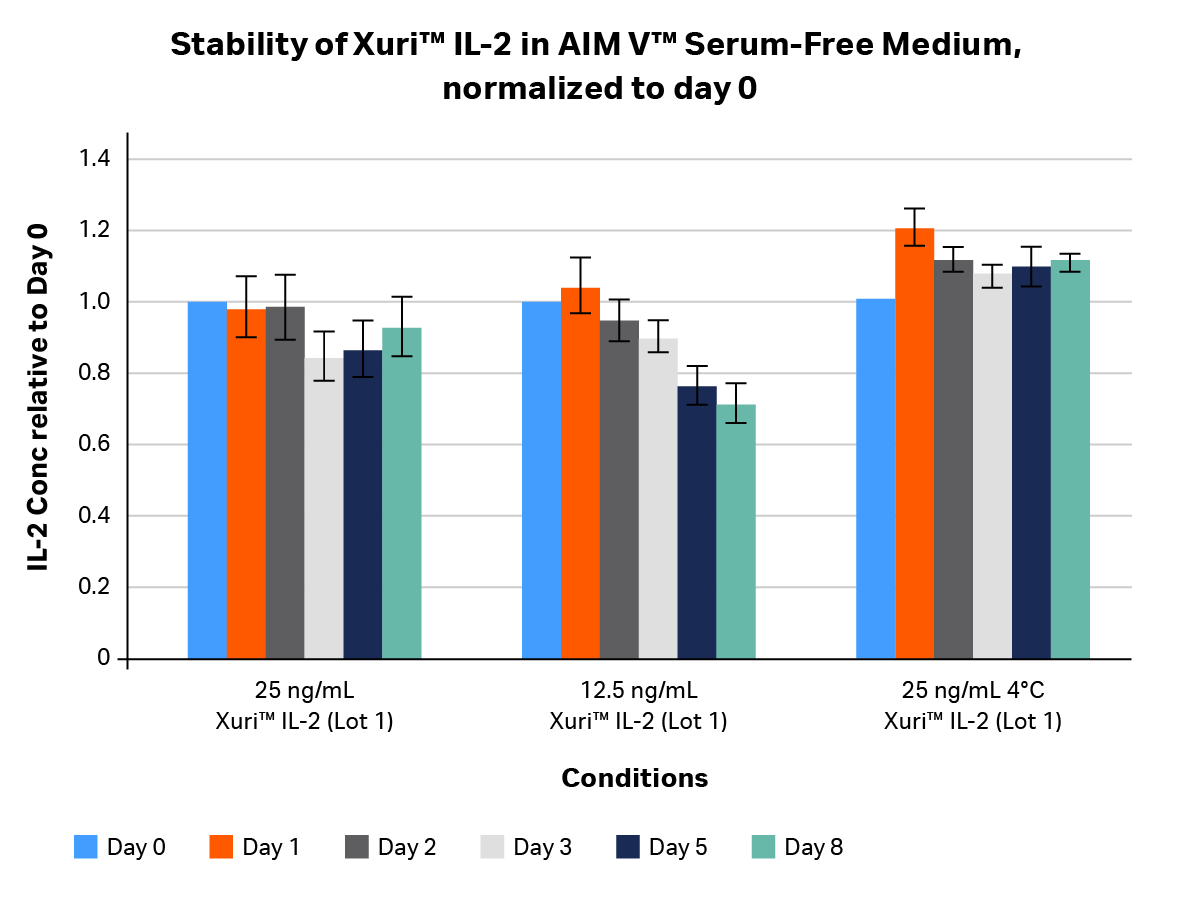
Fig 3. Stability profile of GMP-compliant Xuri™ IL-2 in AIM V™ Serum Free Medium over 8 days, normalized to day 0. Xuri™ IL-2 was added to AIM V™ medium at two different concentrations (25 and 12.5 mg/mL) and kept in a humidified tissue culture incubator at 5% CO2 and 37°C. A control sample of AIM V™ medium containing Xuri™ IL-2 was left at 4°C. Media were collected at days 0, 1, 2, 3, 5, and 8, protease inhibitors added, and samples frozen in a -80°C freezer. After IL-2-contanining media from all time points were collected, frozen samples were thawed and IL-2 concentration was determined using an OptEIA™ IL-2 ELISA kit.
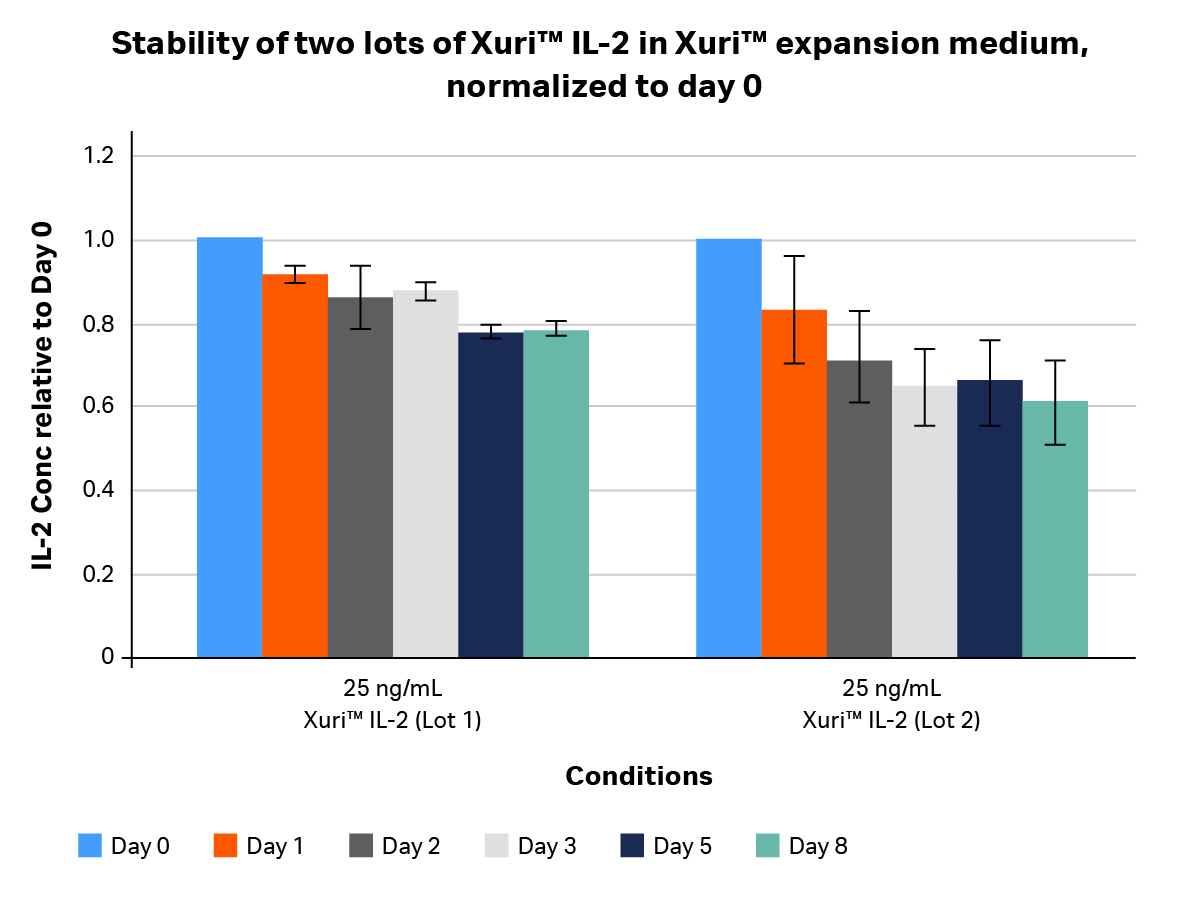
Fig 4. Stability profile of two different lots of GMP-compliant Xuri™ IL-2 in Xuri™ expansion medium containing 5% human AB serum over 8 days, normalized to day 0. Xuri™ IL-2 from each lot was added to human AB serum-supplemented Xuri™ expansion medium at 25 ng/mL on day 0 and kept in a humidified tissue culture incubator at 5% CO2 and 37°C for up to 8 days. Media were collected at days 0, 1, 2, 3, 5, and 8, protease inhibitors added, and samples frozen in a -80°C freezer. After media from all time points were collected, samples were thawed and IL-2 concentration was determined using an OptEIA™ IL-2 ELISA kit.
Discussion and conclusions: Xuri™ IL-2 stability over time
Figure 1 shows data from evaluations of fresh versus frozen samples containing IL-2. Samples cryopreserved at -80°C trended higher in concentration value reading compared to media run fresh on an ELISA plate and this shift was consistent across all groups, however the differences were within a margin of error in nearly all cases. To account for this phenomenon, all subsequent sampling was done using frozen samples and normalized to day 0 readings, and all subsequent data was expressed in relative units rather than absolute concentrations.
At 37°C in serum-supplemented Xuri™ expansion medium over an 8-day period, Xuri™ IL-2 at most showed a 45% drop in concentration, with a < 30% drop by day 8 on average (Fig 2). We observed the same finding with AIM V™ Serum-Free Medium (Fig 3), suggesting the drop was independent of the medium used for reconstitution. This decline in concentration was expected, as most cytokines are not stable at 37°C for more than a few days.
At 4°C, GMP-compliant Xuri™ IL-2 was extremely stable, maintaining its concentration profile until day 8 without any drop.
While we observed an expected drop in Xuri™ IL-2 concentration over time, the product performed well under typical cell therapy manufacturing conditions. When not in use, IL-2 containing medium should ideally be stored at 2-8°C. Even during use, media reservoirs attached to perfusion loops should ideally be refrigerated at 2-8°C as a matter of best practices, and only media that will be used within a few days should be prepared, however practical considerations (e.g. availability or placement of refrigeration equipment, bioreactor perfusion parameters, limited space and/or particulate concerns in GMP cleanrooms) may not always allow for this.
Learn more about Xuri™ growth factors
References
- Weigent DA, Stanton GJ, Johnson HM. Interleukin 2 enhances natural killer cell activity through induction of gamma interferon. Infect Immun. 1983;41(3):992-997. doi:10.1128/iai.41.3.992-997.1983
- Boyman O, Sprent J. The role of interleukin-2 during homeostasis and activation of the immune system. Nat Rev Immunol. 2012;12(3):180-190. Published 2012 Feb 17. doi:10.1038/nri3156
Ordering information
| Product | Format | Pack size | Product code |
|---|---|---|---|
| Xuri™ IL-2 * | Vial | 10 µg | 29062789 |
| Xuri™ IL-2 * | Vial | 1 mg | 29062790 |
| Xuri™ IL-2 * | Syringe | 1 mg | 29295127 |
| Xuri™ IL-15 * | Vial | 40 µg | 29112116 |
| Xuri™ IL-21 * | Vial | 40 µg | 29112119 |
* USP <1043> for further manufacturing only