Filtration in air quality testing
Ambient air quality testing is a critical aspect of environmental safety. Organizations, such as government agencies and businesses, routinely carry out air quality measurements to investigate airborne particulate matter to detect the volume and composition of particulate matter that could have an impact on health.
What is airborne particulate matter?
Airborne particulate matter (PM) is, simply put, air pollution that consists of a mixture of small solid particles and droplets of liquid. Particulates come in all shapes and sizes. The composition of a particulate and its origin (natural or synthetic) contributes to its size and effect on human health.
Particulates smaller than 100 µm are the most relevant to air pollution monitoring, because they have long atmospheric lifetimes and studies have demonstrated their impact on human health.
Standardized measurements for particulate matter include PM 2.5 and PM 10 monitoring, with the number signifying the lower detectable limit (in µm) of the particle diameter.
In general, a PM 10 measurement includes particles that are airborne for a shorter time than PM 2.5, and it includes more naturally occurring particles. PM 2.5 particles are more often synthetic and more hazardous to health.
How is air quality measured?
PM 10 and PM 2.5 monitoring is typically performed by gravimetric methods. Often used in pollution monitoring, PM 10 and PM 2.5 are measured as weight in a given volume of air and are a subset of the total suspended particles in air.
After gravimetric assessment, specific follow-up tests and chemical analysis can provide detailed information on the chemical composition or presence of specifications such as heavy metals in air.
Learn more about Whatman PM2.5 PTFE membranes for continuous air monitoring
How do you ensure collection of a representative sample?
The easiest and fastest way to collect a sample of airborne particulate matter is by using an air filter. This is accomplished by passing air though a filter for a fixed time and at a fixed speed to collect the sample of particulate matter retained on the filter. The selected pore size determines the lower limit of the particles collected. Selecting the right filter is key to ensuring a representative sample, and therefore reliable test results.
A key characteristic of a filter when it comes to testing air quality is its ability to retain a significantly high fraction of particles of a given size on its surface. Retention efficiency directly affects the reliability of air quality sampling and the subsequent gravimetric assessment.
Which filters are most suitable for testing air quality?
A common method to assess retention efficiency and air filter integrity is dioctyl phthalate (DOP) testing with a test particle of known size.
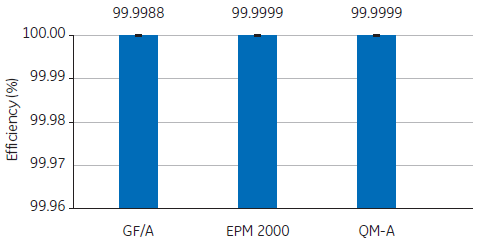
For example, to determine the suitability of three glass fiber filters, a study compared the retention efficiency of Whatman GF/A, EPM 2000, and QM-A using DOP tests. In all three cases, the air filters indicated excellent retention efficiency when presented with a 0.3 µm test particle (Fig 1).
Fig 1. Retention efficiencies of Whatman GF/A, EPM 2000, and QM-A air filters in a 0.3 µm DOP test.
Selecting a filter for further chemical analysis
Advanced testing of collected particulate matter, such as heavy metal analysis of air in environmental assessments, requires filters with minimal amounts of elements of interest to protect against the filter’s composition adversely affecting the analysis. For example,
Small amounts of cadmium, copper, lead, nickel, manganese, zinc, cobalt, or iron, in the filter’s composition can interfere with techniques, used to measure the concentrations of these elements in the air sample.
The study identified the trace element levels for three Whatman glass fiber filters digested by acid and analyzed by ICP-MS (Table 1).
All three filters show exceptionally low levels of many elements, including the heavy metals mercury, cadmium, and arsenic. The data provide a good reference for choosing the most appropriate filter for specific analyses. For example, QM-A is better suited to measurement of lead in airborne particulate matter than EPM 2000 or GF/A, while EPM 2000 is better suited for zinc measurement than either other option.
Table 1. Result of a trace element analysis of Whatman’s QM-A, EPM 2000, and GF-A air filters.
| Trace element | QM-A (ppm) |
EPM 2000 (ppm) |
GF/A (ppm) |
| Antimony (Sb) | 1 | 6 | 1 |
| Arsenic (As) | <1† | <1† | 5 |
| Beryllium (Be) | <1† | <1† | <1† |
| Cobalt (Co) | <1† | 1.2 | <1† |
| Cadmium (Cd) | <1† | <1† | <1† |
| Chromium (Cr) | 3 | 10 | 16 |
| Copper (Cu) | <1† | 5 | 1 |
| Iron (Fe) | 47 | 323 | 223 |
| Lead (Pb) | <1† | 3 | 5 |
| Manganese (Mn) | 2 | 20 | 6 |
| Mercury (Hg) | <1† | <1† | <1† |
| Nickel (Ni) | 1 | 2 | 1 |
| Silver (Ag) | <1† | <1† | <1† |
| Thallium (Ti) | <1† | <1† | 1 |
| Vanadium (V) | <1† | 2 | 1 |
| Zinc (Zn) | 70 | 51 | 25583 |
* Analysis was via complete acid digestion in a microwave and inductively coupled plasmamass spectrometry (ICP-MS).
† Below detectable limits of the test.
Try our Whatman Filter Selector App to find out if you are using the most appropriate filtration solution for air quality sampling. To discuss any challenges you are facing, please contact Cytiva Scientific Support.