Microbial quality testing of environmental water samples, including municipal water sources, public bodies of water, cooling towers, and wastewater, can by nature be difficult work, both during field collection and in the laboratory. Microbiology QC laboratory workflows need to consider efficiency, budget, and regulatory requirements. They also need to limit the possibility of cross-contamination between samples.
The Membrane Filtration (MF) technique is a well-accepted reliable method for fast, flexible testing, whether testing materials with concentrated or very low levels of microbes. The technique was introduced in the late 1950s as an alternative to the Most Probable Number (MPN) procedure for microbiological analysis of water samples. The MF Technique offers the advantage of isolating discrete colonies of bacteria, whereas the MPN procedure only indicates the presence or absence of an approximate number or organisms (indicated by turbidity in test tubes).
Advantages of the Membrane Filter technique include:
- Permits testing of large sample volumes
- Reduces preparation time as compared to many traditional methods
- Allows isolation and enumeration of discrete colonies of bacteria
- Provides presence or absence information within 24 hours
- Allows for removal of bacteriostatic or cidal agents that would not be removed in Pour Plate, Spread Plate, or MPN techniques
The MF technique can be performed by utilizing different laboratory workflows that may implement the use of individual disposable funnels, partially disposable products, or reusable hardware. As previously mentioned the choice of workflow needs to consider laboratory efficiency, budget, and contamination risks and meet regulatory requirements. However, other factors may influence the workflow; these include the number of daily samples, number of laboratory technicians, availability of laboratory space, sample logistics, autoclave size, and personal choice.
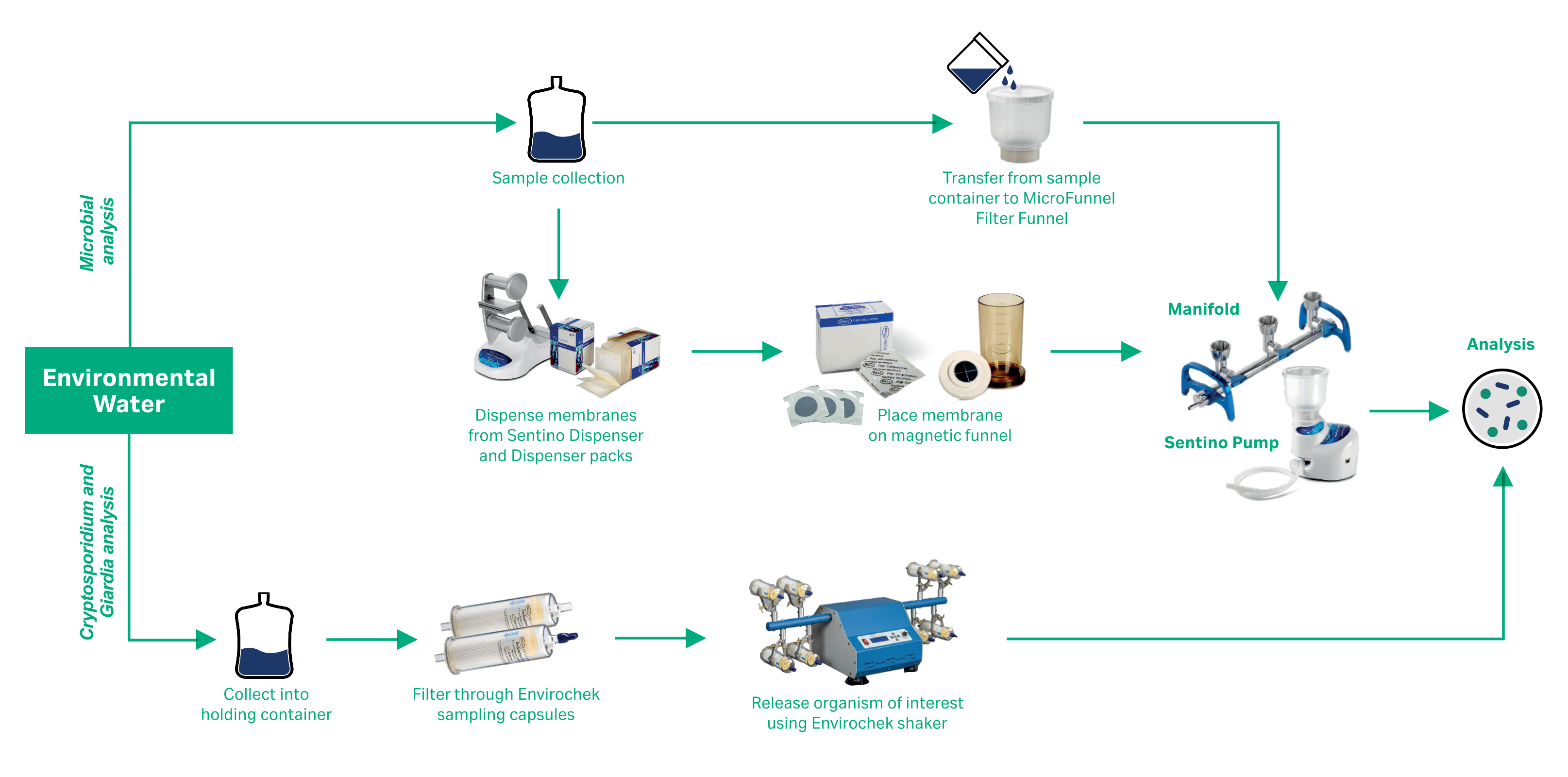
Figure 1 gives examples of environmental water testing workflows utilizing some of the many Cytiva quality control products. We offer tools that provide efficient solutions for high volume sampling, products that can help reduce the risk of sample-to-sample cross contamination and improved ergonomics, all while meeting necessary regulatory requirements.
Fig 1. Workflow examples.
Culturing sensitive organisms can be difficult, and identification is critical for process control and public safety. Analysis membranes must be manufactured to the highest standards for accurate microbial growth and recovery.
We supply membrane disc filters that are available in either individual packs or dispenser packs. Dispenser packs are available for use on our Sentino™ filter dispenser or the eButler membrane dispenser.
Our membranes include the mixed cellulose ester GN Metricel™ membrane, available in a 0.45 µm pore size, and the Supor™ 200 PES membrane, available in a 0.2 µm pore size.
We also supply a number of membranes for specific testing, such as green and black membranes of larger pore sizes for yeast and mold analysis.
Fig 2. Cytiva microbiology products
Sentino™ filter funnels and magnetic funnels
Our membranes can be used with our Sentino funnels or our patented magnetic filter funnels (Pall™ Life Sciences products).
Sentino funnels are available in both 100 mL and 250 mL volume formats. They are gamma irradiated eliminating the need for autoclaving or any other cleaning/sanitizing techniques. The base of the funnel adapts for use with the Sentino™ pump or microbiology manifold.
Cytiva magnetic filter funnels feature a unique magnetic closure action that facilitates a simple one-handed operation while ensuring there is no risk of membrane disk ripping or tearing that can occur with screw fitting funnels. These reusable funnels feature a sturdy plastic construction that is shatterproof and can withstand multiple autoclave cycles. The 47 mm magnetic filter funnels are available in 300 mL and 150 mL sizes for an easy fit into autoclaves. For larger volume samples a 500 mL size is available.
MicroFunnel™ Filter Funnels
An alternative to using separate membranes and funnels is to use MicroFunnel filter funnels (Pall™ Life Sciences products).
Our disposable, gamma irradiated MicroFunnel filter funnels come ready-to-use, fully assembled, with both filter membrane and funnel. Featuring a convenient and ready-to-use design, these disposable filter funnels increase the productivity and efficiency of busy laboratories that do not have time to clean and sterilize reusable hardware.
Microfunnel filter funnel units provide an easy squeeze separation of the funnel from the base, preventing membrane disruption and offering fast, no-hassle operation. The base of the funnel can easily convert to a Petri dish for culturing using the provided funnel lid. Alternatively, the membrane can be easily removed for placing on separate agar dish.
The units are available in either 100 mL or 300 mL volume sizes with either 0.2 µm, 0.45 µm, or 0.8 µm membranes. Each lot is certified for microbiological analysis to provide added assurance of reliable results. They are individually bagged, eliminating potential cross-contamination risks.
Fig 3. MicroFunnel Plus filter funnels
MicroFunnel Plus filter funnel units have further evolved the design of the MicroFunnel filter funnels in order to improve efficiency and offer increased protection from contamination. The MicroFunnel Plus filter funnels feature a vented lid that snaps closed to ensure a liquid-tight seal; this allows for sample collection in the field, transportation, and filtration all in the same unit.
A separate sample cup is no longer necessary for collection, and there is no need to transfer your sample into a disposable funnel for concentration. The vented filter on the lid prevents airborne contamination that could be drawn into the funnel during filtration.
Microbiology manifold and Sentino™ Pump
We provide microbiology manifolds and pumps that can be used in tandem with our MicroFunnel products or alternatively they can be used with separate membranes and funnels.
Our microbiology manifold allows you to optimize testing without sacrificing cleanliness.
- Requires no tools or tape to put together
- Disassembles easily for thorough cleaning
- Inlet and outlet can be set up at either end of the manifold, avoiding the risk of contamination due to reaching over the top of funnels to turn valves on and off
The manifolds are available in a 3-place format, which can easily be coupled together to form a 6-place manifold. The modular design allows for easy separation for disinfection and/or sterilization.
Microbiology Manifold
Coupled Microbiology Manifolds
The Sentino Pump (a Pall™ Life Sciences product) streamlines analysis by replacing the traditional vacuum filtration system with a small peristaltic-action pump that draws samples through the filter funnel. The filtrate is channeled directly to drain or waste collection through a disposable fluid path. The fluid path is simple to load and completely disposable to eliminate potential biofilm build-up. There is no need to clean, wrap, and autoclave a bulky multi-place manifold. The potential for sporadic and unnecessary contamination is removed.
The unit’s compact design and its ability to be operated by a self-contained rechargeable battery means that it’s easy to use in on the benchtop or in the field, providing flexibility for optimal efficiency and workflow.
Fig 6. Sentino™ pump.
The concentration and recovery of Cryptosporidium and Giardia cysts
Envirochek™ capsules (Pall™ Life Sciences products) can be used for the collection and recovery of Cryptosporidium oocysts and Giardia cysts in both finished and source water, including surface water, municipal water supplies, samples in containers, or wells.
When using the Envirochek sampling capsule, the water sample to be tested is passed through the capsule either by sampling at the source or by taking a “grab” sample back to the laboratory and filtering it on the bench top.
The capsule is then filled with an elution solution, placed on a laboratory shaker, and vigorously shaken to elute any captured oocysts and cysts. Up to eight capsules can be processed at once using our laboratory shaker. The elution solution is decanted and centrifuged to a pellet for further examination by the user’s method of choice.
Fig 7. Envirochek™ HV capsule
Envirochek capsules are validated and listed in U.S. EPA Methods 1622 and 1623, and used for sampling source water for Cryptosporidium and Giardia. Together, the EPA method and Envirochek capsule present a major improvement over the previous string wound cartridge method. The Envirochek capsule is also listed in ISO/DIS 15553.
Envirochek HV capsules are designed for sampling up to 1000 liters or more of treated water and validated for up to 50 liters of source water. The Envirochek HV incorporates a track-etched membrane designed to process high volumes of treated water while maintaining high recovery characteristics and meet U.S. EPA requirements. Envirochek HV capsules are also approved by the United Kingdom DWI standard operating protocols for monitoring drinking water for Cryptosporidium.
Reducing repetitive strain injuries in microbiology QC Labs
When performing microbiological testing as well as ensuring laboratory efficiency it is also important to select high-performance products with ergonomics that reduce long-term injury risk.
The repetitive nature of routine laboratory work puts technicians and scientists at risk for repetitive strain injuries (RSI). Routine activities often include repeating the same movements over and over, which can take a toll on hands, wrists, and shoulders and can lead to serious injury. RSI can lead to laboratory user fatigue and variability that causes poor technique, errors, and increase cross-contamination.
In a microbiology laboratory, there are many ways to avoid manual labor stress by working in a more ergonomic manner. Choosing the right tools can make all the difference.
Download our scientific brief – “How Ergonomics and Cleaning Ease Reduce Repetitive Stress Injuries and Contamination in Environmental Quality-Control Lab Workflows.”