Cytiva's modern resins are used routinely for ion exchange chromatography (IEX), affinity chromatography (AC), and size exclusion chromatography (SEC). This document provides a list of journal references where Cytiva's solutions have been used in two- or three-step purification protocols.
Click on the links to see the journal references and summaries for each topic.
Glycoproteins
Altamirano, A. et al. Expression, purification, and biochemical characterization of human afamin. J. Proteome Res. 17(3), 1269–1277 (2018).
https://pubs.acs.org/doi/10.1021/acs.jproteome.7b00867
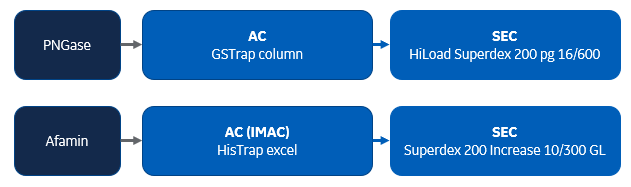
Summary: Afamin is a glycoprotein containing five N-glycosylation sites. This paper describes the expression and purification of afamin having different glycosylation patterns with the goal to elucidate structural data. Wild-type and nonglycosylated rhAFM were purified from CHO cells using 1 mL HisTrap excel columns containing Ni Sepharose resin for affinity chromatography (AC) in a first step. The first purification step was followed by SEC using Superdex 200 10/300 Increase columns. GST-PNGase, used for deglycosylation of afamin, was purified using a 5 mL GSTrap column followed by SEC using a HiLoad Superdex 200 pg 16/600 column.
Glycoprotein multistep purification protocols used in this publication:
DNA-binding proteins
Murayama Y. et al. Establishment of DNA-DNA interactions by the cohesin ring. Cell 172 (3), 465–477, (2018).
https://www.cell.com/cell/fulltext/S0092-8674(17)31501-5
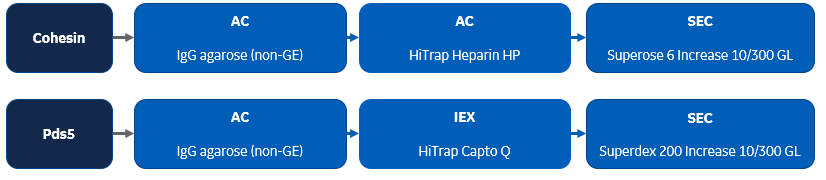
Summary: This paper describes how the ring-shaped structural maintenance of chromosome (SMC) complexes (multisubunit ATPases) encircle DNA. Fission-yeast proteins were purified using IgG agarose, HiTrap Heparin HP, and Superose 6 Increase 10/300 GL by using sequential column chromatography. Pds5 was purified using IgG agarose as well as HiTrap Capto Q and Superdex 200 Increase 10/300 GL columns. Subsequent DNA capture experiments with the purified proteins were performed.
DNA-binding protein multistep purification protocol used in this publication:
Virus proteins
Roymans, D. et al. Therapeutic efficacy of a respiratory syncytial virus fusion inhibitor. Nat. Commun. 8, article number 167 (2017).
https://www.nature.com/articles/s41467-017-00170-x
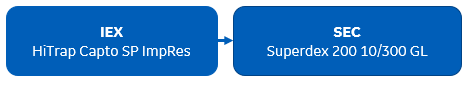
Summary: This paper reports the structure as well as biophysical data for a novel anti-viral inhibitor bound to respiratory syncytial virus fusion (F) protein in its prefusion conformation. The prefusion RSV F virus protein was purified using a two-step purification starting with IEX using HiTrap Capto SP ImpRes column followed by SEC using a Superdex 200 column. The binding of the protein was characterized in biophysical experiments (isothermal titration calorimetry and differential scanning fluorimetry). For crystallization trials, protein was digested overnight with thrombin to remove the tag, followed by SEC using Superose 6 column.
Virus protein two-step purification protocol used in this publication:
Antibodies, Fab fragment
Chodorge, M. et al. A series of Fas receptor agonist antibodies that demonstrate an inverse correlation between affinity and potency. Cell Death Differ. 19, 1187–1195 (2012).
https://www.nature.com/articles/cdd2011208
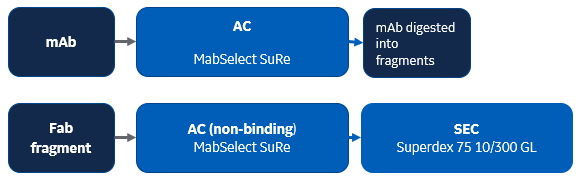
Summary: The paper describes the crystal structure of the anti-FAS agonist antibody complex. Anti-Fas E09 human IgG1 was purified by protein A chromatography using MabSelect SuRe resin. IgG was then digested and MabSelect SuRe column was used to purify Fab fragments in a nonbinding mode. This was followed by a size exclusion step using Superdex 75 column. According to a Biacore surface plasmon resonance (SPR) binding assay, binding activity of the E09 Fab fragment was shown to be similar to the human IgG1.
Multistep purification protocol used in this publication:
Vaccines
Krarup, A. et al. A highly stable prefusion RSV F vaccine derived from structural analysis of the fusion mechanism. Nat. Commun. 6, article number 8143 (2015).
https://www.nature.com/articles/ncomms9143?origin=ppub
Summary: Respiratory syncytial virus (RSV) can lead to acute lower respiratory tract infections and in this paper the purification of a vaccine antigen has been described. The recombinant polypeptides were purified by a two-step protocol applying IEX using HiTrap Capto S followed by an additional purification step using Superdex 200 column from Cytiva.
Two-step purification protocol used in this publication: