What is hydrophobic interaction chromatography?
Hydrophobic interaction chromatography (HIC) separates proteins according to differences in their surface hydrophobicity. HIC utilizes a reversible interaction between the proteins and the hydrophobic ligand of a HIC resin.
The interaction between hydrophobic proteins and a HIC resin is greatly influenced by the running buffer. A high salt concentration enhances the interaction. Lowering the salt concentration weakens the interaction.
How does hydrophobic interaction chromatography work?
Proteins with different degrees of surface hydrophobicity can be separated using hydrophobic interaction chromatography. The proteins are bound to the hydrophobic ligand on the HIC resin in a binding buffer with a high salt concentration.
When the ionic strength of the buffer is reduced, the interaction is reversed. Therefore, the protein with the lowest degree of hydrophobicity is eluted first. The most hydrophobic protein elutes last, requiring a greater reduction in salt concentration to reverse the interaction.
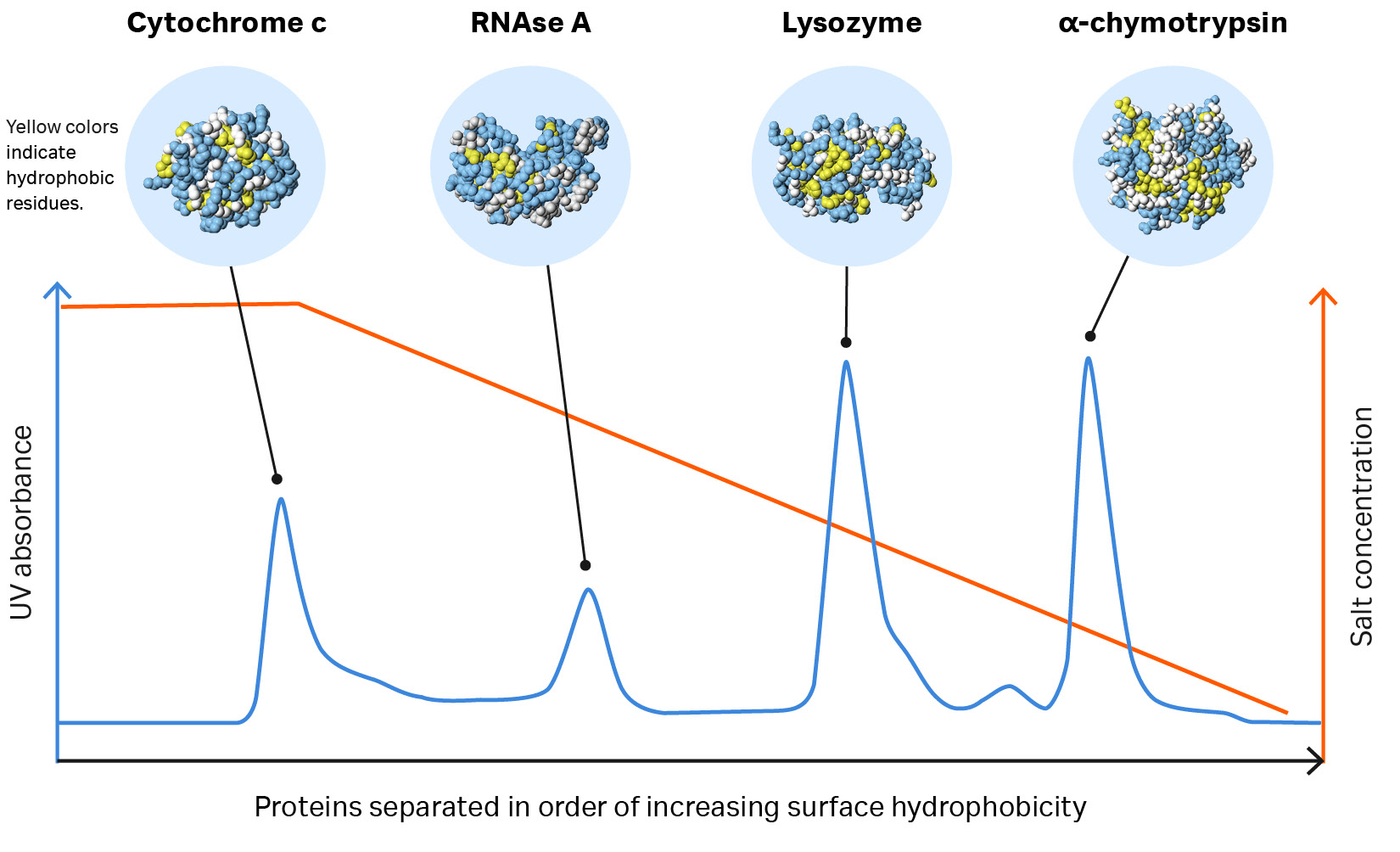
Fig 1. Proteins separate according to differences in their surface hydrophobicity (yellow indicates hydrophobic amino acid residues and blue indicates hydrophilic amino acid residues) as shown in this separation of standard proteins on one of Cytiva’s HIC resins.
You can perform hydrophobic interaction chromatography either in bind/elute, or in flow-through mode. Table 1 shows how you use HIC in four main steps in bind/elute mode. In bind/elute, the target molecule binds to the ligand coupled to the resin through hydrophobic interactions. Changes in the buffer composition and pH release the molecule from the resin to allow collection (the molecule elutes with the buffer).
Table 1. Four main steps of a hydrophobic interaction chromatography that you can perform in bind/elute mode
| Equilibration | Sample application and wash | Elution | Regeneration |
| Prepare the column to the desired start conditions.
Equilibrate HIC resin with high-salt start buffer. |
Target molecule binds to the ligand on the resin via hydrophobic interactions. The sample buffer needs to have the same pH and ionic strength as the starting buffer. |
Decreasing salt content (using a linear gradient) causes hydrophobic proteins to elute. The least hydrophobic proteins elute first. | Removes all molecules still bound to the stationary phase. Ensures the full capacity of the resins is available for the next run. 0.5 to 1.0 M NaOH is often used to clean the resin. |
In flow-through mode, impurities bind to the resin while the target molecule is collected in the chromatography flowthrough.
Watch our animation to easily visualize how hydrophobic chromatography works:
When should I use hydrophobic interaction chromatography?
Hydrophobic interaction chromatography is a versatile technique that can be used for capture, intermediate purification, or polishing steps.
The major advantage of HIC is providing selectivity that unavailable with affinity or IEX resins. HIC is suitable for purification of a large range of molecules:
- recombinant proteins and peptides
- monoclonal antibodies (mAbs), bispecific mAbs, and antibody-drug conjugates (ADC)
- mRNA
- plasmids
HIC is particularly well suited for capture steps after sample cleanup by ammonium sulfate precipitation or for intermediate steps directly after an ion exchange separation where the sample is already in a high-salt solution.
How does reversed phase chromatography (RPC) differ from HIC?
Reversed phase chromatography also separates proteins and peptides on the basis of hydrophobicity. Compared with a HIC resin, the surface and ligands of an RPC resin are usually more hydrophobic. This increased hydrophobicity leads to stronger interactions that, for successful elution, must be reversed using nonpolar, organic solvents such as acetonitrile or methanol.
As many proteins are denatured by organic solvents, RPC is not generally recommended for protein purification. Instead, RPC is well-suited for applications such as peptide mapping or purity checking.
How do I determine optimal experimental conditions?
We recommend you explore a wide range of chromatography conditions early to increase process understanding and increase the likelihood of developing a robust purification process. The use of high-throughput process development (HTPD) plates facilitates the choice of resin and initial conditions for column chromatography.
This article outlines a step-by-step HTPD workflow to determine the optimal resin and initial conditions for HIC.
What’s the difference between bind and elute and flow-through modes?
Bind and elute mode, also known as step elution, is used when you want to retain your target protein on the column and subsequently elute it. The binding of the target protein to the HIC resin is typically achieved under high-salt conditions. After loading the sample, the non-specifically bound contaminates are removed with washing the column under high-salt conditions. The elution of the target protein is achieved by reducing the salt concentration in the elution buffer in a linear manner or a step gradient manner.
Flow-through mode is used when you want to retain the impurities on the column while allowing your target proteins to pass through the column unbound. It is useful when the target protein in the elution condition has low hydrophobicity and thus interacts weakly with the HIC resin.
The choice between bind and elute and flow-through modes depends on the specific characterization of your target protein and the impurities to be removed. If you are not certain which mode to use, we recommend starting with the bind and elute mode to scout the relative hydrophobic interaction of the target protein, impurities, and the resin. Then decide whether flow-through mode will be feasible for your purification.
How do I determine optimal experimental conditions?
We recommend you explore a wide range of chromatography conditions early to increase process understanding and increase the likelihood of developing a robust purification process. The use of high-throughput process development (HTPD) plates facilitates the choice of resin and initial conditions for column chromatography.
This article outlines a step-by-step HTPD workflow to determine the optimal resin and initial conditions for HIC.
What’s the difference between bind and elute and flow-through modes?
Bind and elute mode, also known as step elution, is used when you want to retain your target protein on the column and subsequently elute it. The binding of the target protein to the HIC resin is typically achieved under high-salt conditions. After loading the sample, the non-specifically bound contaminates are removed with washing the column under high-salt conditions. The elution of the target protein is achieved by reducing the salt concentration in the elution buffer in a linear manner or a step gradient manner.
Flow-through mode is used when you want to retain the impurities on the column while allowing your target proteins to pass through the column unbound. It is useful when the target protein in the elution condition has low hydrophobicity and thus interacts weakly with the HIC resin.
The choice between bind and elute and flow-through modes depends on the specific characterization of your target protein and the impurities to be removed. If you are not certain which mode to use, we recommend starting with the bind and elute mode to scout the relative hydrophobic interaction of the target protein, impurities, and the resin. Then decide whether flow-through mode will be feasible for your purification.
What are some tips for getting started with HIC?
- Before the purification, check the stability of protein at the pH and salt concentrations that you intend to use.
- Screen salt type, pH, and conductivity for the optimal running conditions
- For samples with unknown hydrophobic properties, try the following buffer conditions:
- Start buffer: 1.5 M ammonium sulfate, 50 mM sodium phosphate, pH 7.0
- Elution buffer: 50 mM sodium phosphate, pH 7.0
- Save time and sample by using HTPD tools such as 96-well plates prepacked with different resins for a quick and efficient screening of various resins and buffer conditions for your required selectivity.
- To further save time and create more robust processes, consider using mechanistic modeling. Mechanistic models use computer simulations to decrease the number of experiments.
How do I select hydrophobic interaction resins and columns?
Overview of Capto™ HIC resins
Capto™ HIC resins are based on a rigid, high-flow agarose base matrix with an optimized pore structure that offers good pressure and flow. These resins are intended for use in process development and large-scale manufacturing and in research labs. Their high flow rates allow increased productivity and large-volume processing.
Table 2. List of Capto HIC resins
| High-flow and high-capacity resins | High-resolution resins |
|---|---|
Capto PlasmidSelect
Purified plasmid DNA is required in increasing quantities to meet emerging requirements for gene therapy and DNA vaccine applications. Capto PlasmidSelect resin is a thiophilic aromatic adsorption chromatography resin with a selectivity that allows supercoiled, covalently closed, circular forms of plasmid DNA to be separated from open circular forms. The resin is designed for the purification of high quality, supercoiled DNA for gene therapy and DNA vaccine applications.
Hydrophobicity
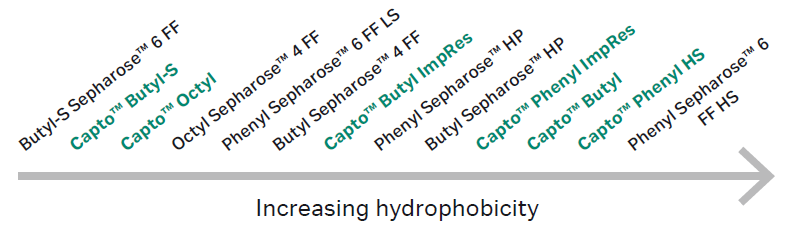
Figure 2 displays the relative hydrophobicities of Capto phenyl (high sub), Capto butyl, Capto octyl, and Capto butyl-S resins
Fig 2. Relative hydrophobic scale of various resins based on retention of ribonuclease A, lysozyme, and α-Chymotrypsin. Hydrophobicity can change with running conditions and proteins. The resins highlighted in green are the Capto HIC resins, which enable optimized productivity compared to Sepharose™ based resins.
Resin selection
To select the most suitable resin for your specific needs, we recommend starting with one these kits:
- PreDictor™ Capto HIC screening kit: a set of 96-well plates prefilled with six Capto hydrophobic interaction chromatography resins for use in high-throughput process development
- HiTrap™ Capto HIC selection kit: a set of five Capto HIC resins in HiTrap columns for resin selection and condition screening
Prepacked formats available for Capto HIC resins
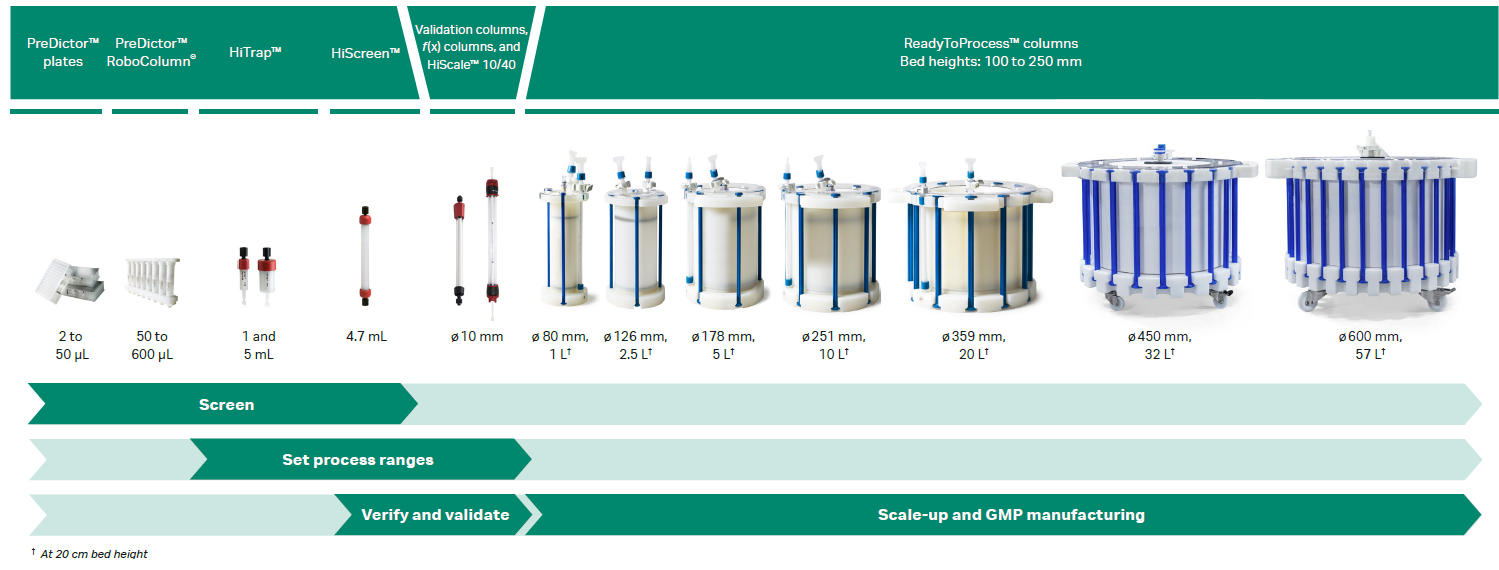
Our resins are available both in prepacked formats (Fig 3) and as bulk resins to be packed in columns for use from lab-scale to large-scale GMP manufacturing.
Fig 3. Overview of prepacked formats
Application notes and packing guidance
- Developing a HIC polishing step for removal of mAb aggregates
- Increasing productivity in hydrophobic interaction chromatography (HIC) using Capto resins
- Optimization of a hydrophobic interaction chromatography step for recombinant protein purification
- How to pack Capto HIC resins using verified packing methods
- High-throughput screening of HIC media in PreDictor plates for capturing recombinant Green Fluorescent Protein from E. coli
- High-throughput screening and process development for capture of recombinant pro-insulin from E. coli