Ion exchange chromatography: overview
Ion exchange chromatography (IEX) separates proteins with differences in surface charge to give high-resolution separation with high sample loading capacity. The separation is based on the reversible interaction between a charged protein and an oppositely charged chromatography resin. Ion exchange chromatography resins can be used at high flow rates, because binding kinetics for IEX are fast, and rigid chromatography particles can be used.
For in-depth information about IEX, download our IEX handbook.
How does ion exchange chromatography work?
The net surface charge of proteins varies according to the surrounding pH. The pH at which a protein has no net charge is called isoelectric point (pI). Above its isoelectric point (pI), a protein will bind to a positively charged anion exchanger. Below its pI, a protein will bind to a negatively charged cation exchanger.
Proteins bind as they are loaded onto a column at low ionic strength. The conditions are then altered so that bound substances are desorbed differentially. Elution is usually performed by increasing salt concentration or changing pH in a gradient.
IEX is performed in four main steps as shown below. Watch our IEX animation to easily visualize how IEX works.
| Equilibration | Sample application and wash | Elution | Regeneration |
|---|---|---|---|
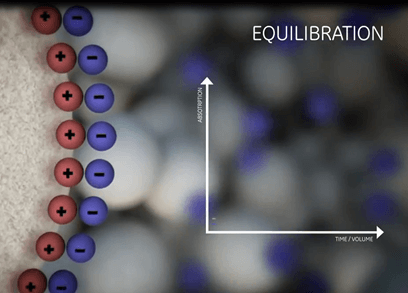 |
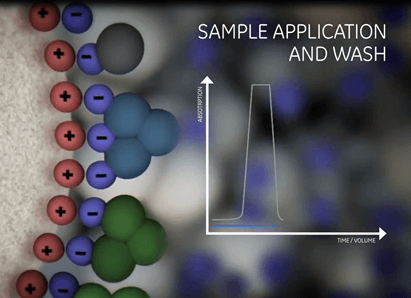 |
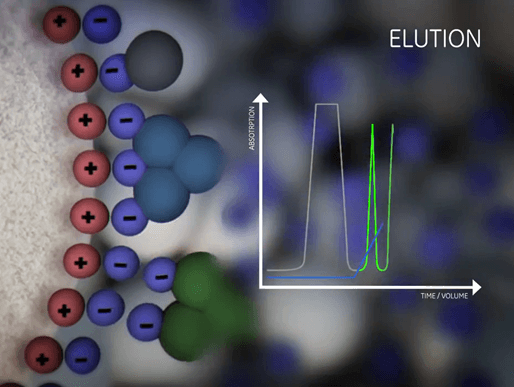 |
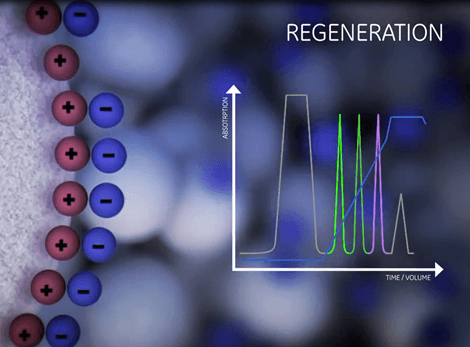 |
| Prepare the column to the desired start conditions. | Bind the target molecules and wash out all unbound material. | Biomolecules are gradually released from the ionic exchanger by a change in the buffer composition. | Removal of all molecules still bound. |
When should I use ion exchange chromatography?
Ion exchange chromatography can be used in any part of a multistep purification procedure.
Download this guide that will show you when and why an IEX step is recommended for a powerful purification protocol.