Molecular imaging enables the visualization and understanding of biological molecules taking place at a cellular and subcellular level. The molecular imaging detection of biological molecules on gels, blots and arrays can be performed using a wide variety of labelling and staining techniques in combination with the appropriate imaging device or imaging system. Therefore, it is important to first consider the quality and quantity of data required from your experiments. For example, finding out whether a protein is present in a sample or simultaneously detecting multiple proteins down to picogram quantities will determine which type of workflow, imaging system, method and detection you use.
To explore the history of advanced medical imaging and molecular imaging systems, click here.
Molecular imaging systems: detection
What is detection? -Explore our “7- Step guide to Western Blots” here.
Protein detection for low abundance proteins has resulted in increased requirements for sensitivity and linear dynamic range when using techniques such as gel- and/or blot-based quantitative analysis. A wide linear dynamic range is particularly important for applications where weak and strong signals are compared in the same experimental series.
Components of imaging systems such as the excitation source (for fluorescent imaging), lens, emission filter, detector and the basic technical principles used (e.g. confocal optics, moving scanner head or galvanometer technology) all impact linear dynamic range and image quality. Digital resolution (signal intensity levels distinguished by the imager) and spatial resolution (related to photosite configuration, as well as the number of lines per millimeter that can be resolved in an image) are also important.
CCD camera-based systems are composed of an illumination source and optics that focus the image onto a CCD chip. They are area imagers that integrate chemiluminescent signals or fluorescent signals from a continuously illuminated sample field. Most of these systems are designed to capture a single view of the imaging area, using lens assemblies with either a fixed or variable focal length. Some flatbed scanners, are also CCD camera-based and capture the illuminated section during a scan cycle.
In addition, the most appropriate choice of label or stain will also enhance performance in terms of both sensitivity and dynamic range. Fluorescent labels and stains can be more sensitive and have a broader dynamic range than colorimetric stains. These are important considerations when large variations in signal intensity are expected.
Types of molecular imaging detection
What is a fluorescence detector?
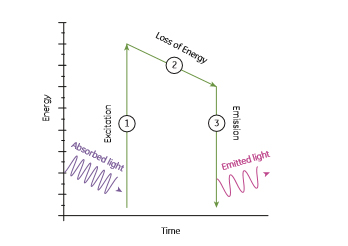
The light phenomenon of fluorescence occurs when molecules called fluorophores absorb light. In their ground state, fluorophores do not emit light, but when subjected to light (excitation) their energy levels are raised to a brief but unstable excited state. As fluorophores return to their ground state, they emit light at a lower energy, (longer wavelength) than the excitation light (Fig 1.1).
Fig 1.1: Fluorescence can be summarized as a 3-step process: (1) Excitation of a fluorophore at ground state, by absorption of light, (2) a short period in an excited but unstable state that usually relaxes towards the lowest vibrational energy level within the excited state before, (3) returning to ground state with the emission of light at a lower energy and longer wavelength than the absorbed light.
Fluorescence is a regenerative process; fluorophores can repeatedly undergo excitation, which means that a fluorophore can produce a signal several times, making this method potentially very sensitive and stable. However, photodestruction or photobleaching, is the effect that results from the enhanced chemical reactivity of the fluorophore when excited. Since the excited state is generally much more chemically reactive than the ground state, a small fraction of the excited fluorophores molecules can participate in chemical reactions that alter the molecular structure of the fluorophore, thereby reducing fluorescence. The rate of these reactions depends on the sensitivity of the particular fluorophore to bleaching, the chemical environment, the excitation light intensity, the dwell time of the excitation beam or time of exposure, and the number of excitation cycles.
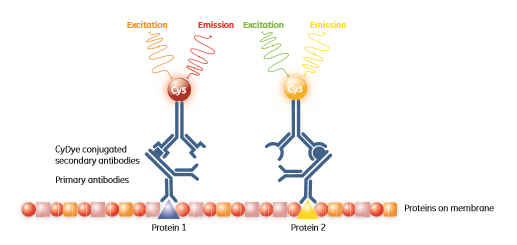
Proteins can be pre-labelled with fluorescent dyes that are spectrally resolvable to allow for multiplexing, with the resulting ability to accurately quantitate changes in expression. The ability to multiplex can also be used in Western blotting where different proteins can be targeted by antibodies conjugated to different fluorescent dyes (Fig 1.2).