All our chromatography resins are designed, verified, manufactured, tested, and released for delivery, according to the Cytiva global quality management system (QMS), to ensure their proper and consistent function.
The Cytiva product lifecycle is governed by standardized requirements and quality procedures for continuous product improvements. Cytiva’s business is certified according to the ISO 9001 and ISO 22301 standards and operates under Cytiva global QMS.
Our customers manufacture biopharmaceuticals, which is a large commitment to the public health and helps save the lives of millions of people. The manufacturing is complex and the end product is dependent of the use of equally consistent, high-quality key manufacturing components. Chromatography resin is an example of a key manufacturing component.
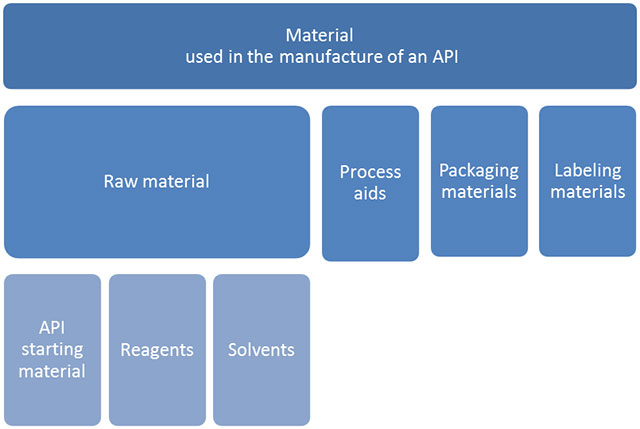
Different types of materials are used in the manufacture of biopharmaceuticals and APIs. An API is any substance or mixture of substances intended to be used in the manufacture of a pharmaceutical product and that becomes an active ingredient of the pharmaceutical product by its pharmacological activity or other direct effect in the diagnosis, cure, mitigation, treatment, or prevention of disease or to affect the structure and function of the body.
The term raw material is used for API starting materials, reagents and solvents intended for use in the production of API. API starting material is incorporated as a significant structural fragment into the structure of the API. Process aids are materials used in the manufacture of an API that do not themselves participate in a chemical or biological reaction (filter aid, activated carbon, etc.). It is our conclusion that a chromatography resin is to be regarded as a “process aid”, used for the manufacture of an API, but not defined as a raw material. (ICH Q7)
The Cytiva product lifecycle is governed by standardized requirements and quality procedures. Our policy for chromatography resin supply is to not discontinue any product that is used in an approved, registered process without customer consent. Our policy also states that if a discontinuation should be decided, a three-year notification period should be used when there are change control notification (CCN) subscribers.
The CCN service is an essential component in the product lifecycle, changes made to BioProcess resin are published to all subscribers of the CCN service.
Access the policy for discontinuation of BioProcess chromatography resins and single-use products.
Being one of the world’s leading suppliers of chromatography resin, Cytiva’s business has knowledge to meet the high-quality demands of work in regulated environments. We also offer regulatory support documentation for products used in regulated environments.
Regulatory support files (RSF)
Documentation for BioProcess chromatography resin. Detailed information on performance, stability, extractable compounds, and analytical methods.
Change control notification (CCN)
A service that supplies information regarding changes that might potentially impact your product, process, documentation, or procedures. It provides information as a basis for your evaluation of the change.
Certificate of analysis (CoA)
Certificates detailing release criteria for BioProcess chromatography resin.
Material safety data sheets (MSDS)
Search our complete database of material safety data sheets (MSDS).
General animal origin information
Uppsala manufacturing and R&D site is registered at the Swedish Board of Agriculture as an establishment for management of animal by-products and derived products according to regulation (EC) No. 1069/2009, laying down health rules as regards animal by-products and derived products not intended for human consumption. For TSE/BSE relevant species products comply with EMA 410/01 current revision (European Medicines Agency, Note for Guidance on minimizing the risk of transmitting spongiform encephalopathies via human and veterinary medicinal products.)
Safe sourcing as defined in regulation (EC) No. 1069/2009 is applied for all animal-derived chemicals used as raw materials or in the manufacturing process of BioProcess chromatography media. Safe sourcing implies using only material that possesses no unacceptable risk to the public and animal health.
To gain reliable information we work closely with our raw material suppliers to gather information about the source of raw materials. When possible, material of animal origin is excluded or substituted. For animal-derived materials the following information is always requested:
- Species of animal used
- Tissue of animal used
- Country of origin of animals
- Compliance with EMA Note for guidance 410/01 when applicable
- Certification that animal-derived material is sourced from healthy animals fit for human consumption, that is, category 3 material according to regulation (EC) No. 1069/2009.
- Information to show safe sourcing of human materials should be provided.
Container materials
The primary packaging materials comply with Code of Federal Regulations 21 CFR 177, indirect food additives; polymers, from FDA.
REACh
Cytiva actively monitors and acts upon regulatory requirements for chemical compounds and articles, to enable business sustainability and continuous supply to our customers.