Host cell protein (HCP) management is critical to biologics development – and getting it wrong could delay your development cycle. Learn how the right HCP strategy can enhance your route to market.
CHO cells: Making biologics
As a biologic manufacturer, chances are that Chinese hamster ovary (CHO) cells are your workhorse.
CHO cells are now involved in the production of over 70% of recombinant biopharmaceutical products, mostly monoclonal antibodies, with trastuzumab (Herceptin) and rituximab (Rituxan) being some high-profile examples (1).
Cultured since the 1960s, CHO cells are prolific (pardon the pun) in research, and have been approved for use as a mammalian expression system for biotherapeutics for several decades. Part of what makes them so suitable for biologics manufacture is this history – they are well-characterized and easy to culture.
However, even with CHO cells, you are likely to face a few challenges on your route to market.
One of these challenges is host cell protein (HCP) analysis. Cell lines used in biologics manufacture produce HCPs as biological by-products, and CHO cells are no exception. These HCPs can potentially influence the efficacy and toxicity of your drug, and have long-term immunogenicity.
So, it is no wonder that regulatory bodies have rules and suggested strategies for HCP analysis and reporting, and it is likely that removing HCPs is a key concern in your process development. Ultimately, the accuracy of monitoring and success of purifying your biologic of these HCPs can make or break your product.
Find out about the advantages of using a specific CHO HCP ELISA kit validated for high coverage

The challenge of analyzing HCPs
HCPs are complex. For one thing, scientists use a variety of biological expression systems to produce pharmaceutical drugs – systems based around bacteria, yeast, mammalian cells, and plant cells – and each system has a unique HCP profile.
Even if you are using a well-characterized CHO cell line, the HCP profile will change in response to different growth conditions and manufacturing processes. Each step of the purification process will also affect the HCP profile, making the effects of changes to that process hard to predict.
U.S., European, and Japanese pharmacopeia require scientists to measure HCPs at each stage of the drug development process: pre-clinical, prior to the toxicology assessment, at each clinical trial phase, and so on.
A general expectation is to reduce HCPs to < 100 ng/mL of final product as measured by the enzyme-linked immunosorbent assay (ELISA). But given their complex nature and how different HCPs can have greater or lesser effects, there is no exact guidance on acceptable HCP limits.
To be meaningful, these measurements need to be as accurate and reliable as possible, and preferably backed by orthogonal assays like mass spectrometry to identify key HCPs. Authorities can then make an honest assessment of the safety and efficacy of each product on a case-by-case basis.
For a manufacturer, the consequences of not meeting the requirements — whether through insufficient or inaccurate data — are significant. At best, you could face additional costs and process development steps — or, at worst, you could have your drug held back, route to market delayed, and clinical trial stopped (2).
What about analyzing HCPs in biosimilars?
If you develop and manufacture biosimilars, it is easy to think of your product being so like the out-of-patent original that all this extra fuss is unnecessary. But the same rules apply, as well as the risks of using inaccurate or unreliable HCP assays.
Even if you are using the same expression system as the original biologic’s manufacturer, every component from media to purification will be slightly different. You need to effectively treat your biosimilar like a new drug developed for the same purpose as the original.
Is my HCP assay good enough?
ELISAs are the most common method for measuring HCPs, but they rely on the affinity and selectivity of antibodies, and those properties can vary. In other words, you are unlikely to be able to detect every HCP in your sample.
Sometimes this might be because the levels of specific HCPs are below the threshold for reliable detection. Other times you might simply not have antibodies raised against some HCPs because they were not immunogenic enough to trigger a response. This is where coverage assays come in.
Coverage assays are a regulatory requirement and provide a cross-check of the effectiveness of your HCP ELISA, so many manufacturers conduct them as a matter of course, rather than risk the additional costs and inconvenience of delays on their route to market.
The 2016 U.S. Pharmacopeia (USP 39, <1132>) discusses coverage assays in section 3.2.2.2. Although there is no clear guidance on the minimum acceptable level of ELISA coverage, industry players generally consider 50% the minimum, and > 70% preferable.
Confirming that your ELISA sensitivity can achieve the top end of this range can depend on your approach to coverage assays.
Which HCP ELISA coverage assay should I use?
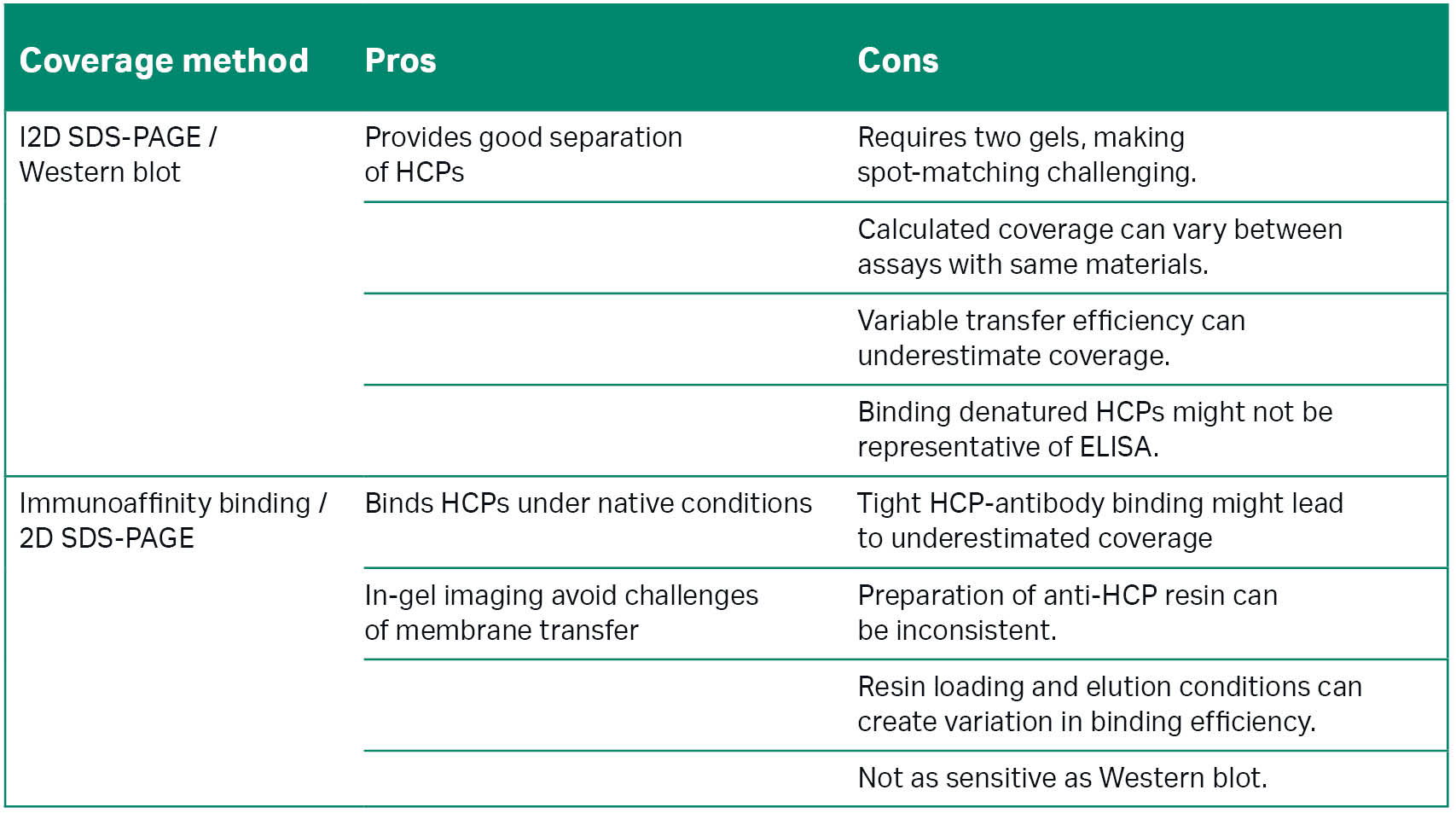
There are several methods for assessing HCP ELISA coverage. Traditionally, most manufacturers used 2D SDS-PAGE/Western blot, and many still do. It is a relatively straightforward protocol based on long-established chemistries.
The U.S. Pharmacopeia compares the merits of 2D SDS-PAGE/Western blot with immunoaffinity binding/2D SDS-PAGE, a comparatively recent but more complex approach. Figure 1A and 1B highlight key differences in these approaches and Table 1 provides a summary of their advantages and disadvantages.
Table 1: Comparison of 2D SDS-PAGE/Western blot and immunoaffinity binding/2D SDS-PAGE for HCP coverage assays. Information adapted from the 2016 U.S. Pharmacopeia (2).
Affinity and DIGE vs 2D SDS-PAGE/Western blot for HCP coverage assays
Essentially, immunoaffinity binding, alternatively known as using affinity and differential in-gel electrophoresis (DIGE), captures HCPs using a chromatography column containing resin-bound anti-HCP antibodies. Scientists label the captured proteins with Cy5, mix them with Cy3-labeled total protein, and compare them in-gel after 2D electrophoresis.
Imaging these gels with standard gel and blot imaging systems then enables you to use image analysis software to accurately identify where Cy5 and Cy3 spots overlap and calculate percentage coverage.
A key advantage to this approach is that, unlike Western blots, anti-HCP antibodies target the HCPs in native conditions. DIGE also means you can use a single gel, saving time and effort, and not be concerned about matching spots across multiple gels or blots.
But switching from the Western blot approach is not as straightforward as printing out a new protocol. Staff must be trained to prepare the resin consistently, and be wary that it might not be as sensitive as an immunoblot.
Simplify HCP assay analysis with Melanie Coverage software
DIBE: An improved coverage assay approach
Differential in-blot electrophoresis (DIBE) is an alternative to both affinity with DIGE and 2D SDS-PAGE coverage assay methods that provides many of the benefits with few of the drawbacks.
The DIBE workflow involves labeling the entire sample with Cy3 to detect total protein, rather than splitting the sample to label one half with Cy3 and affinity capture and label the other with Cy5. This approach avoids some of the complexity and variability found in affinity and DIGE.
Running the Cy3-labeled sample on a single 2D gel and transferring to a membrane then enables you to use a more sensitive immunoblot to test the anti-HCP antibodies. The workflow is much the same a Western blot, but rather than HRP, a Cy5 secondary reagent provides a clear, amplified signal for imaging.
Smoothing the route to market with optimized assays
Given HCP analysis is receiving increased attention from regulatory bodies, it makes sense for manufacturers to take any action that could minimize the bumps in the road for biologics.
If you are using CHO cells for biologics or biosimilar production, the greatest effect might come from simply using a commercially available ELISA that is validated to provide high coverage.
Similarly, pairing the HCP ELISA with an affinity and DIGE or DIBE coverage assay would help maximize the reliability and accuracy of coverage data. If you are currently using the 2D SDS-PAGE/Western blot approach for this, switching to DIBE is straightforward.
For support with optimizing your HCP analysis, contact our Scientific Support team, or your local Cytiva representative.
Learn about our end-to-end solution for HCP analysis
- Why, why, why… ELISA? A look at the benchmark HCP assay (blog)
- How well are your HCP ELISAs covered? (blog)
- Enhanced host cell protein analysis in biologics manufacturing (article)
- Robust HCP coverage analysis with dedicated Melanie software (PDF poster)
- Challenges of host cell protein analysis with ELISA (webinar)
- Lalonde, M.E. Therapeutic glycoprotein production in mammalian cells. Journal of Biotechnology 251, 128-140 (2017)
- US Pharmacopiea. USP 39 NF 34 Residual Host Cell Protein Measurement in Biopharmaceuticals. US Pharmacopieal Convention (2016)