Towards a scalable and cost-efficient process
The viral vector market is highly active, and the interest in production technologies is driven by recent approvals in cell and gene therapy as well as vaccines. Here I share insights around the challenges in viral vector manufacturing. The focus is on helping anyone who is developing a viral vector process to obtain a scalable and cost-efficient process suitable for good manufacturing practice (GMP) manufacturing environments.
The demand for viral vectors
The main driver is the large pipeline in different clinical stages and scales. In the last quarter of 2023 there were 598 clinical trials for gene therapies and 520 for cell therapies (1), many of which are based on viral vectors, heavily focused on adeno-associated viruses (AAV) and lentiviruses. Use of AAV has grown rapidly over the last few years, mostly in the gene therapy area. Development of oncolytic virus therapies and vaccines are driving a lower demand for other viral vectors, particularly adenovirus.
Compared with use in cell therapy, high-dose systemic administration for gene therapy requires a larger quantity of infectious virus. In addition, clinical trials are expanding beyond rare diseases to prevalent ones that affect a greater number of people (1). Together, these realities place higher demands on process efficiency and production scale.
What to consider in process development
Early on, we suggest thinking about whether your process can be scaled up easily and whether it will ultimately be compatible with GMP environments. Consider that making changes to your manufacturing process during clinical trials will likely require comparability studies for regulatory submission.
Developing a process ― including analytics — with the end in mind will facilitate a robust process that minimizes batch-to-batch variation. Such foresight may also avoid costly delays later if the process must be reworked. If you’re looking for long-term success, it’s important to take the time early on to get the process design right.
The complexity challenge
Viral particles are much more complex than molecules such as monoclonal antibodies (mAbs) or other recombinant proteins. First, viral vectors are derived from several virus types, each with its own biological properties. And viral vectors can be produced by transfection with plasmids, stable packaging or producer cell lines, or infection using virus stocks. The large virus particles pose challenges during chromatography, and enveloped viruses are sensitive to processing conditions including shear force. Because of this complexity, it’s challenging to find platforms for cell lines as well as upstream and downstream virus production.
The virus characteristics, as well as the program goals, will influence the choice of cell line and conditions. The biological properties of the virus will also affect how you develop the purification process.
Scalable processing
As a first step, think about the intended population size you’re targeting and the dose size. For autologous cell therapy applications, production scales are several liters. The scale will be much larger for allogeneic (off-the-shelf) cell therapies. Volumes above 1000 L might be required for high-dose systemic gene therapy applications or for large populations.
Lab-scale methods, such as culturing in 2D flatware, cell lysis by freeze-thawing, and purification by ultracentrifugation are not easy to scale up. We suggest replacing them with cell culture in suspension, filtration, and chromatography methods, respectively.
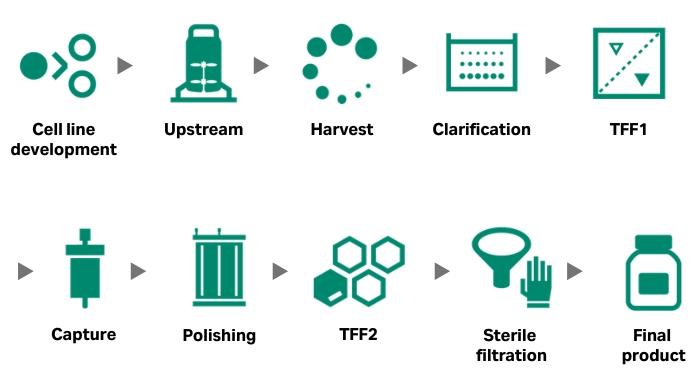
A general workflow for virus production and purification is shown in Figure 1.
Fig 1. Generic workflow for a virus production and purification process, including cell line development.
Regulatory requirements
We expect regulatory requirements to increase and get more standardized as viral vector-based therapeutics gain greater adoption and reach larger populations. Purity demands are high and increasing and are especially challenging for high-dose therapies. AAV-based gene therapies, which are administered in vivo, require a high percentage of full capsids with low levels of impurities such as host cell DNA (hcDNA).
Regulatory authorities are concerned about cross-contamination and adventitious agents. This concern can be mitigated with single-use technology, which also allows aseptic and closed processing. The use of animal origin-free raw materials is encouraged. Also, all buffer components must be compatible with clinical manufacturing. Regulatory authorities are looking for you to show that you have controlled the sources of variation in your process. So, it’s important to have a robust overall process, including in the purification steps that must accommodate any variations in feed material. It’s a good idea to engage with regulatory agencies early to smooth your path to submission. Learn more about viral vector regulatory considerations.
Analytical challenges
Physical titer, infectious titer, and impurity levels are common measurements during process development and manufacturing. Product- and process-related impurities need to be diminished to acceptable levels. AAV preparations must contain a high percentage of full capsids containing all required genetic elements.
Analytics for viral vectors are time-consuming and important for your process, but they’re not always robust. Some analytics have high variation and low accuracy. Without robust analytics, you can’t control variation in your process ― because you can’t measure it accurately. Another thing to think about is that your assays might be influenced by the impurity levels of your samples and your buffer compositions. To confirm results, several different assays are routinely used for the same target. Orthogonal analytics are recommended to confirm the purity of AAV full capsids. Finally, consider whether developed methods are suitable for GMP environments. Figure 2 shows one such method, surface plasmon resonance, for titer analysis.
Fig 2. Viral vector titer analysis using a Biacore™ system.
Upstream processing
Viruses can be propagated using either transient transfection, packaging cell lines, or producer cell lines. Host cell lines are typically mammalian, but baculovirus systems using Sf9 insect cells are an option for AAV.
Transient transfection is a common way to introduce elements for viral production and the gene of interest (GOI) into mammalian cells. The host cell line can be cultured in adherent mode or in single-cell suspension. To ensure efficient virus propagation and a robust upstream process when using transient transfection, make sure to optimize parameters such as plasmid ratio, total DNA, mixing time with the transfection reagent, and time of harvest.
There is growing interest in using packaging or producer cell lines in mammalian cells to stably produce the virus, eliminate costly plasmids, and enhance robustness. Stable production is particularly suitable for prevalent indications, where required volumes are very high. Before deciding on a cell line and viral propagation method, consider your program goals.
Single-use bioreactors are widely used for viral vector production. Options suitable for GMP environments include stirred-tank bioreactors for suspension growth (Fig 3) and fixed-bed bioreactors for adherent cells. The advantages over stainless steel are faster change-over between batches and lower risk of cross-contamination. Single-use bioreactors can also help you make your facility more productive and flexible.
Fig 3. Upstream processing in an Xcellerex™ X-platform single-use bioreactor.
To align with regulatory expectations, well-characterized and documented cell lines are essential for virus propagation. Also, the plasmids and other materials needed for this step must have high quality for GMP manufacturing. As mentioned previously, it’s a good idea to avoid all animal-derived components such as serum and to use chemically defined cell culture media.
Optimizing cell culture and virus propagation is worthwhile to maximize performance and avoid rework later. Regulatory and process economy demands can be met by using modern and scalable upstream technologies.
Downstream processing
One point to remember throughout your purification process is to maintain virus infectivity, if it’s needed for your application. Remember that virus stability can be affected by buffer salt and pH. If you have problems with virus stability, you can add stabilizers.
To maximize overall process economy, it’s important to optimize the recovery of each downstream step and minimize the number of steps. A combination of filtration and chromatography steps is typically used, as shown in Figure 1.
When cells are harvested from the bioreactor, most processes include cell lysis to release the virus particles inside. If your process requires this step, be sure to use a detergent that’s compliant with all of the regulatory requirements. For example, Triton X-100 isn’t allowed for large-scale production in Europe anymore, for health and environmental reasons. In addition to freeing virus particles, cell lysis releases host cell DNA (hcDNA), which is challenging to remove. DNase treatment reduces the length of this impurity to allow its removal. Notably, hcDNA that’s present inside capsids is protected from DNase digestion.
The crude harvest will contain a high level of impurities, including cellular debris. Effective clarification is essential for achieving efficient downstream processes. Centrifugation may be used at lab scale. However, this method is difficult to scale up. We recommend depth filtration (Fig 4) to maximize process efficiency and recovery. Following clarification, main contaminants to remove include empty viral capsids, hcDNA, and host cell protein. Partially filled capsids, with the wrong DNA or truncated DNA, can also be an issue.
Fig 4. Depth filtration of viral vectors using an Allegro™ MVP system.
During filtration steps, shear forces should be minimized to maintain viral integrity and infectivity, if required. Ensure gentle processing for sensitive viruses such as lentivirus. Hollow fiber filters have been shown to reduce shear, so they are well-suited for tangential flow filtration (TFF) steps in viral vector workflows.
Virus characteristics impact the chromatography steps. First, the large virus particles can only bind to the outer surface of the resin bead or chromatography support, which has a negative impact on the binding capacity. We recommend using chromatography membranes or modern chromatography resins with smaller beads to improve capacity compared with legacy resins. Also, virus particles are large and have multiple points of attachment to a chromatography support. For those reasons elution conditions can be difficult to predict and typically require more optimization than for protein-based processes.
In conclusion, purification must be optimized at each step to maximize recovery and impurity removal. Regulatory and process economy demands can be met by using scalable and modern downstream technologies, such as the chromatography system shown in Figure 5.
Fig 5. Downstream processing of viral vectors using an ÄKTA ready™ 450 system.
Learn more about scalable viral vector processes
AAV: AAV vector production workflow | Cytiva (cytivalifesciences.io)
Lenti: Lentivirus production | Cytiva (cytivalifesciences.io)
Adeno: Adenovirus production | Cytiva (cytivalifesciences.io)
References
1. The Alliance for Regenerative Medicine. Q4 2023 cell and gene therapy sector data (2023). Accessed 5 March 2023. https://alliancerm.org/publication/2018-annual-report/. Accessed online March 14, 2024.
About the author
Åsa Hagner McWhirter, PhD is a Principal Scientist in Viral Vectors Downstream Bioprocessing R&D. Åsa has been with Cytiva based in Uppsala, Sweden since 2003 and is a downstream and analytics SME, with a broad and deep understanding of viral vector processing. Due to her long experience and from customer interactions she has gained insights into common challenges and pitfalls for processing and analytics of viral vectors and vaccines. She has been leading teams developing innovative new tools to improve performance and productivity of viral vector processing. Åsa holds a PhD in Medical Biochemistry from Uppsala University in 1999 based on research around biosynthesis of proteoglycans.