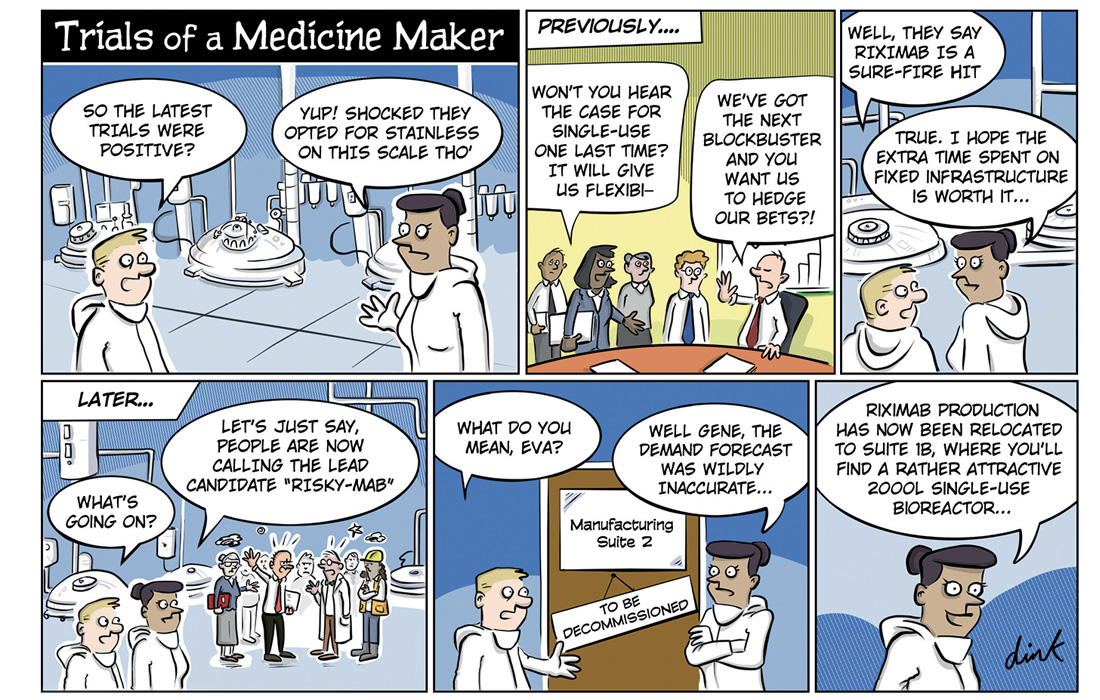
Wouldn’t it be great to be able to see into the future? That way you could decide your biomanufacturing strategy early on. As we continue to dream about this unlikely scenario, let’s join Gene and Eva as they look at the next best thing.
Ouch! A risky mAb is not what you wish for that late in the drug development journey. The good news is that you can actually avoid some of the negative impacts of a situation like this. How? One way would be exploring alternative biomanufacturing approaches early in the development phase.
It might sound backwards in an industry where stakes are so high, but stepping out of the comfort zone of tried and tested solutions can be a way to decrease risk. And, at the same time, speed up time to market.
Single-use technologies (SUTs) might still seem like the new kid on the block for some of us, compared to stainless-steel solutions. The truth is that SUTs are now part of many commercial manufacturing processes, simply because they can help decrease capital risks, allowing faster implementation, lower upfront investment, and increased flexibility.
The way forward: transparency, transparency, and transparency
Remember that you’re not in this alone. SUTs bring vendors and manufacturers closer than ever. And by exchanging experiences and information across “borders,” the future of biomanufacturing is bright. Want to know more? Check out this e-book where industry leaders share their single-use insights.
Thanks to the open communication, a massive amount of experience is building up across the industry. So, make sure to leverage it. Patrick Guertin, Global Technical Manager at Cytiva, shares his thoughts on converting to single-use technologies in this short video:
Any thoughts on what SUTs can bring to your process?