FAQ
How can I optimize a run with CleanGel IEF?
You may need to extend the IEF time. In order to determine when the correct focusing position has been reached you can apply the samples at both the cathodic and anodic end. Also prefocusing the gel before sample application may improve the result. Due to some drift of the gradient you may not reach exactly the same positions for all the bands when applying them from different side but you will at least get a hint about the focusing time needed. You can divide the sample amount with a factor of two but should be optimized for each sample. When staining it is a good idea to fix the gels over night in order to decrease the background.
Definition of %T and %C
The composition of any polyacrylamide gel is given by two parameters:
%T = concentration of total acrylamide
%T = [g (acryl + bis) / 100 ml] x 100
%C = % of the total acrylamide that is crosslinker
%C = [g (bis) / g (acryl + bis)] x 100
The total percent of acrylamide (%T) in the separating gel, which can range from 5 to 20%, determines the pore size. The percent of crosslinker (%C) used is generally 2.6% for all gels. The stacking gel has a very low total acrylamide concentration because the largest possible pore size is required. A typical stacking gel is 4% T, 2.6% C.
Running procedures
1. Clean the platinum electrode wires, before and after each electrophoresis run, with a wet tissue paper.
2. Place the electrode holder with the IEF electrodes on the electrophoresis unit and align the electrodes so that they rest on the outer edges of the gel. Electrode wicks between the gel and the electrodes are not necessary.
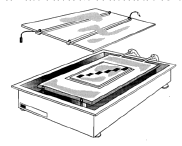
3. Connect the cables of the electrodes to the unit (Fig 6).
Fig. 6 CleanGel IEF placed on the Multiphor II. Samples applied at various positions across the gradient
4. Place the safety lid in position. Connect the power supply. Follow the recommended electrical settings for prefocusing given in Table 2.
Table 2. Recommended running conditions for one CleanGel IEF pH gradient 3 to 10.
| Voltage (V) | Current (mA) | Power (W) | Time (min) | |
|---|---|---|---|---|
| Prefocusing | 700 | 12 | 8 | 20 |
| Sample entrance | 500 | 8 | 8 | 20 |
| Isoelectric focusing | 2000 | 14 | 14 | 90 |
| Band sharpening | 2500 | 14 | 18 | 10 |
Note: If only half of a gel is used, remember to divide the current and power settings by two.
When running narrow pH gradients e.g. pH 5 to 7, use the same electrical settings, but prolong the isoelectric focusing time to 4 hours.
5. Prefocus for 20 minutes without samples in order to establish the pH gradient in the gel.
1. Connect the Multiphor II electrophoresis unit to a thermostatic circulator (e.g. MultiTemp III) and set the temperature to 10°C (15°C for IEF with urea).
2. Switch on the thermostatic circulator 15 minutes before starting the analysis. Isoelectric focusing has to be performed at a defined constant temperature, as the pH gradient and the isoelectric points are dependent on the temperature.
3. Position the gel on the cooling plate.
Note: Wear clean gloves to avoid contamination of the gel surface, particularly when using sensitive silver stain.
4. Pipette about 2 ml of insulating fluid (kerosene or light paraffin oil) onto the cooling plate of the Multiphor II.
5. Remove the gel from GelPool and dry the gel surface with the edge of a filter paper (fig 5).
Fig 5. Drying the gel surface with the edge of a filter paper.
Note: The gel surface should be absolutely dry, otherwise the gel will extrude water during the isoelectric focusing.
6. Position the gel in the centre of the cooling plate, use the screen print as a guide. No air bubbles should be trapped beneath the gel.
Note: If water has been extruded from the gel during the prefocusing procedure it should be removed, see Fig 5.
Fig 5. Drying the gel surface with the edge of a filter paper.
In IEF there is, for most samples, one optimal position within the pH gradient for sample application. This should be found by applying the samples at various positions across the gradient, using sample application pieces (Fig 6).
Fig 6. CleanGel IEF placed on the Multiphor II. Samples applied at various positions across the gradient
In IEF there is, for most samples, one optimal position within the pH gradient for sample application. This should be found by applying the samples at various positions across the gradient, using sample application pieces (Fig 6). There are three different methods for sample application. The optimal method is determined primarily by the sample and the volume to be applied.
- IEF/SDS applicator strip
Up to 52 samples can be applied with a sample volume of 5 to 20 μl in each well. The applicator strip is made of silicon and is applied directly on the gel surface.
- Sample application pieces
Recommended sample volumes 15 to 20 μl, (or for smaller volumes cut the paper). The sample application pieces are made of Paratex. These sample application pieces are valuable when the samples are to be applied at different positions on the gel.
- Very small sample volumes (e.g. 2 μl) can be applied as droplets directly onto the gel surface.
When the protein concentration is between 1 to 3 mg/ml apply 10 μl /sample, by using an applicator strip or sample application pieces.
The pH gradient can be determined by using pI markers (under native conditions).
For determination of the calibration curve: Use a piece of graph paper with 1 cm markings and 0.1 cm subdivisions, position the gel over the grid so the origin of the grid is lined up with the cathode end of the gel. Using the grid, determine the distance from the cathode to each protein pI marker. Plot the known pI value of each pI marker protein versus its distance from the cathode. Connect the points to obtain the pH gradient profile calibration curve.
Place the electrode and safety lid in position (Fig 6) and start the isoelectric focusing according to table 2.
Table 2. Recommended running conditions for one CleanGel IEF pH gradient 3 to 10.
| Voltage (V) | Current (mA) | Power (W) | Time (min) | |
|---|---|---|---|---|
| Prefocusing | 700 | 12 | 8 | 20 |
| Sample entrance | 500 | 8 | 8 | 20 |
| Isoelectric focusing | 2000 | 14 | 14 | 90 |
| Band sharpening | 2500 | 14 | 18 | 10 |
Note: If only half of a gel is used, remember to divide the current and power settings by two.
When running narrow pH gradients e.g. pH 5 to 7, use the same electrical settings, but prolong the isoelectric focusing time to 4 hours.
1. Open the gel package and remove the gel (fig 1)
Fig 1.
2. Select the appropriate reswelling chamber of the GelPool, depending on the gel size.
3. Clean the GelPool thoroughly with distilled water and let it dry. Do not use organic solvents.
4. Degas the rehydration solution in order to remove CO2 for sharper bands in the alkaline region.
5. Pipette the rehydration solution into the chamber, along the left edge of the gel tray.
The following volumes should be used
• Gel, full size (260 x 125 mm) 11 ml
• Gel, half size (130 x 125 mm) 5.5 ml
6. Set the edge of the gel film, with the gel surface downwards, into the rehydration solution and slowly lower it (Fig 2)
Fig 2.
7. Lift the short edge at the middle with forceps and lower the gel down again to get an even distribution of liquid. Avoid trapping air bubbles (Fig. 3).
Fig 3.
8. The gel must float freely in the gel solution. This should be checked by touching the backside of the film with a finger and moving the gel in a circle. This is important for IEF, because of the small volumes.
9. Place the GelPool on a rotary shaker and shake slowly. This is recommended for an even rehydration and to avoid the gel sticking to the GelPool.(Fig. 4)
Fig 4.
10. Lift the gel edge repeatedly during the first 15 minutes to prevent the gel sticking to the GelPool in order to obtain an even rehydration of the CleanGel.
11. After 60 minutes the gel has reswollen completely to a thickness of 0.43 mm, and can be removed from the GelPool, and used at once.
12. If additives e.g. urea or non-ionic detergents are used, rehydration time must be prolonged, up to several hours.
Table 2. Recommended running conditions for one CleanGel IEF pH gradient 3 to 10.
| Voltage (V) | Current (mA) | Power (W) | Time (min) | |
|---|---|---|---|---|
| Prefocusing | 700 | 12 | 8 | 20 |
| Sample entrance | 500 | 8 | 8 | 20 |
| Isoelectric focusing | 2000 | 14 | 14 | 90 |
| Band sharpening | 2500 | 14 | 18 | 10 |
Note: If only half of a gel is used, remember to divide the current and power settings by two.
When running narrow pH gradients e.g. pH 5 to 7, use the same electrical settings, but prolong the isoelectric focusing time to 4 hours.
Note: All chemicals should be of the highest purity. Double distilled water should be used.
Rehydration solutions for pH gradient 3 to 10:
IEF Native
1.1 g Sorbitol (10% w/v)
820 μl Pharmalyte pH 3 to 10
Make up to 11 ml with distilled water.
IEF with Urea
5.28 g urea (8 mol/l)
820 μl Pharmalyte pH 3 to 10
Make up to 11 ml with distilled water.
For other pH gradients see table 1
Table 1. Examples of pH gradients obtained with Pharmalyte.
| pH gradient | Carrier ampholyte mixture | Amount (ml) of Pharmalyte to 11 ml rehydration solution |
|---|---|---|
| 4.0 to 6.0 |
Pharmalyte 2.5 to 5 Pharmalyte 4.5 to 5.4 |
0.56 0.18 |
| 3.5 to 6.5 |
Pharmalyte 2.5 to 5 Pharmalyte 4 to 6.5 |
0.56 0.18 |
| 3.5 to 7.5 |
Pharmalyte 2.5 to 5 Pharmalyte 5 to 8 |
0.37 0.37 |
| 4.0 to 8.0 |
Pharmalyte 4.0 to 6.5 Pharmalyte 5 to 8 |
0.37 0.37 |
| 5.0 to 9.5 |
Pharmalyte 5 to 8 Pharmalyte 8 to 10.5 |
0.37 0.37 |
| 6.0 to 10.0 |
Pharmalyte 8 to 10.5 Pharmalyte 5 to 8 Arginine |
0.59 0.14 22 mg |
Troubleshooting
Find solutions to product related issues. For unlisted issues please contact local Cytiva service representation.
Select symptom:
| Possible cause | Suggested remedy |
|---|---|
Improperly stored gels |
Improperly stored gels will pick up moisture from the air, and subsequently will not rehydrate evenly. Use CleanGels immediately after opening the package. The CleanGel can be cut while still dry, and only a portion used. The unused portion should then be sealed in an airtight plastic bag and stored in a freezer at -20 °C. |
Insufficient rehydration |
1. Ensure that the proper volume of rehydration solution was applied to the GelPool |
Gel was not rehydrated evenly |
1. Improperly stored gels will pick up moisture from the air, and subsequently will not rehydrate evenly. Use CleanGels immediately after opening the package. The CleanGel can be cut while still dry, and only a portion used. The unused portion should then be sealed in an airtight plastic bag and stored in a freezer at -20 °C. |
| Possible cause | Suggested remedy |
|---|---|
Glycerol or sucrose or organic solvent in sample |
Glycerol or sucrose or organic solvent in sample, leading to osmotic distribution. Prepare sample without glycerol or sucrose. Remove organic solvent with a SpeedVac. |
Water condensation on the gel due to high humidity and/or high room temperature. |
Apply the sample on the gel when the cooling plate is still at ambient temperature. Connect cooling plate to thermostatic circulator after sample application |
| Possible cause | Suggested remedy |
|---|---|
Electroendosmosis causes flow of water in direction of the electrodes (especially the cathode) |
This is normal and is not a problem unless it interferes with the run (such as causing the middle of the gel to dry out and burn). Occasionally blot the electrode strips. |
Excessive volume of electrode solution in the electrode strips |
After soaking the strips, blot them with filter paper. |
The electrode solution is too concentrated |
Use at the specified concentration, and dilute if necessary. |