The blockbuster drug era is over, and drug manufacturers are now striving for a more diversified portfolio by targeting a greater number of monoclonal antibody (mAb) products at lower production volumes. With an increased focus on lowering drug prices, though, the key to driving down the overall costs of these therapies is making them with the same or lower capital expenditure investment. Major changes to the landscape of today’s industry are creating higher demands for flexible solutions that can minimize changeover time between campaigns. In addition, high titers from upstream and a surge in established single-use technologies (SUTs) are putting the spotlight on gaps in the process chain where other SUT options could be added to maximize productivity. With a newer toolbox of options to intensify downstream manufacturing operations, it is important to understand what factors should impact your decision.
The changing landscape of biopharma
Over the last couple of decades, bioprocessing has used its existing technologies as efficiently as possible. For example, bioprocessing operations and the cost of goods for manufacturing current blockbuster products are typically well optimized. This means, if the product has a long life cycle, then the fixed assets used for manufacturing are depreciated effectively, achieving approximately $100 to $300 per gram of product, dependent on conditions. (1) Nevertheless, in any business or process, the goal is to identify where efficiency is poor. Considering the evolving landscape described above, where capital expenditures need to be depreciated over lower volume manufacturing batches, costs of goods could increase as much as tenfold, as the number of less-efficient processes is set to grow significantly in the next few years. Now, the focus of drug manufacturers and suppliers is providing new technologies to enable bioprocessing strategies that maximize output from existing and new infrastructure/capital expenditures.
With this future in mind, there are three core areas driving process intensification. The first is drug manufacturers’ move away from their reliance on one blockbuster product to a more diversified portfolio with a greater number of mAb products. With smaller targeted patient populations, more locally sited manufacturing facilities, and, in some cases, the growth of personalized medicine, production volumes will become lower. This requires infrastructure that can easily switch among different product manufacturing processes, without significant downtime, to ensure capital is being depreciated efficiently. For example, a small facility that costs $300 million to build and has a 10-year life span will have $30 million in depreciation each year. If product volumes are so low that enough product can be produced in one batch, the next product can be started faster, creating an opportunity for greater asset utilization (and, thus, a lower attribution of depreciation value to the cost of goods for that product).
The second is a drive toward whole facility utilization, as capital expenditure investments are still the largest contributor to the cost of goods (often over 50 percent). By making more kilograms of mAbs for the same or even lower cost, the investment needed to run a facility will go down, too, leading to lower drug prices.
The third is the well-established issue relating to risk of capital investment. The industry struggles with decisions related to capacity and demand. Build a facility too soon, and there is a risk the product fails and the capacity is not needed; build later when there is a better idea about the success of a molecule and risk the facility not being completed in time. This could result in an inability to meet the market’s demands, leading to a drug shortage and, ultimately, long-term damage to the company’s image. The existing regulatory environment prevents timelines from being easily modified. The choice, then, becomes to delay investment and build faster (potentially achievable with prefabricated formats, such as a KUBio facility) or to not delay investment and instead build a facility that intends to operate in a much more flexible manner with multiple products and campaigns in mind. These reasons and more are why it is critical to be aware of any options that can not only help realize greater facility utilization and cost reduction but also can be used to develop new process strategies for the future, particularly as precision medicine gains traction.
Downstream operations to enhance process economy
Depending on existing infrastructure, batch sizes, and facility utilization, there are different solutions to improve productivity and efficiency in downstream operations.
Improved chromatography resins
When it comes to the capture step, Protein A chromatography resins have proven to be efficient, reliable, and easy to implement at any scale. They have a long track record in the industry and are well accepted by regulatory authorities. However, Protein A resins have historically been more sensitive to harsh cleaning conditions than other chromatographic techniques, due to the nature of the protein ligand. This imposes challenges and risk of microorganism contaminations, as the first capture step is exposed to feed with a lot of nutrients and, at the same time, is sensitive to high concentrations of sodium hydroxide (a common cleaning solution). Later-generation protein A resins have become more alkaline stable, allowing for better bioburden control.
As titers increase, the most common strategy to improve productivity of the batch process will be to increase column size. This requires more use of protein A chromatography resins, though, which can be costly. An alternative response is to use higher-capacity resins, such as MabSelect PrismA. This protein A chromatography resin with enhanced alkaline stability and binding capacity can run up to 80 grams per liter, which decreases process time and reduces resin and buffer consumption.
The high alkaline stability also enables an opportunity to increase the lifetime of the resin by eliminating the drop in binding capacity over repeated cycling. For example, MabSelect PrismA has been demonstrated to run 0.5 molar sodium hydroxide in up to 300 cycles with over 92 percent dynamic binding capacity remaining. The combination of the improved binding capacity and alkaline stability can improve overall process economy of the protein A step by expanding the lifetime of the resin and reducing cost per batch up to 75 percent (Fig 1;2).
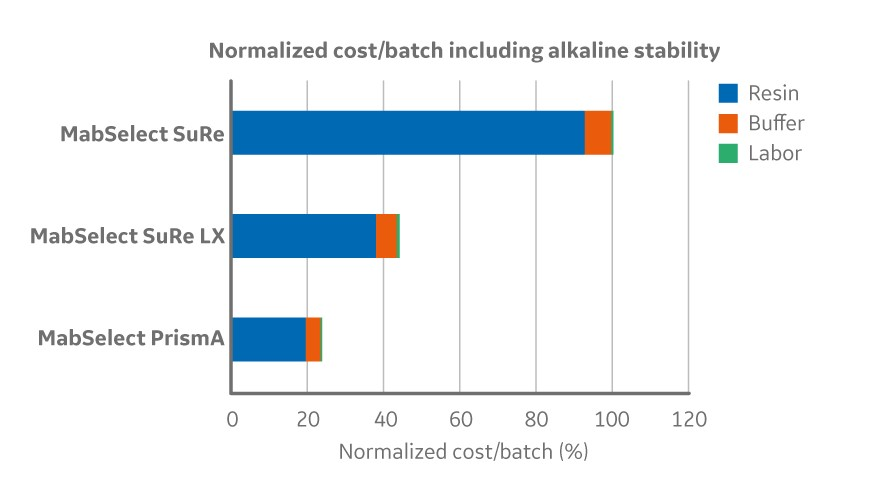
Fig 1. Normalized cost/batch (2000 L bioreactor with titer 2.5 g/L) and resin lifetime for MabSelect PrismA and its predecessor products factoring in alkaline stability (0.5 M NaOH). Lifetime per cycle was measured until 90% of the initial DBC remained when cleaning with 0.5 M NaOH.
Single-use technology and prepacked chromatography columns
SUT is an efficient way to intensify bioprocessing in many circumstances, as it offers several benefits that facilitate switching between product lines in multiproduct facilities, such as limited prequalification work and the elimination of cleaning validation. Prepacked chromatography columns have been on the market for more than a decade, where they started off on a smaller scale. Larger columns have been launched as the use of SUT increased over time. For example, ReadyToProcess columns from Cytiva are now available with an inner diameter of 600 millimeters, allowing the operator to plug and play their purification processes with high titer bioreactor harvests of up to 2000 liters.
Single-use is attractive for not only the columns but also from a hardware system perspective realizing the full potential of disposable techniques. The ÄKTA ready and the recently launched larger ÄKTA ready XL systems demonstrate how the use of disposable flow paths with prepacked columns can enable flexibility and increased speed in bioprocessing. These single-use liquid chromatography systems, built for process scale-up and manufacturing, are comprised of an ÄKTA ready chromatography unit, UNICORN software, and disposable flow paths, including detection flow cells.
While prepacked columns can be used in a single-use mode over one upstream batch, this does result in a high-cost unit operation. Instead, prepacked columns are typically used over a number of batches, reducing labor-intensive setup time but still requiring cleaning and validation between batches. Prepacked protein A columns have been implemented widely in clinical phase production and will likely continue to be implemented in commercial manufacturing, especially for multiproduct facilities where fast changeover rate between campaigns is important.
Continuous chromatography operations
Another way of improving chromatography steps that has been widely discussed across the industry is the use of continuous operations. Many companies have considered hybrid approaches, where only the upstream and downstream are operated continuously, as opposed to an end-to-end flow-through. There is also the option of operating a perfusion bioreactor in combination with a batch purification process or a fed-batch bioreactor upstream followed by a continuous chromatography capture step. Regardless of the approach, the advantage of continuous bioprocessing is reduced cost, improved productivity, and the potential for improved quality. This is due to smaller columns and bioreactors, allowing for reduced buffer consumption and overall facility footprint. In mAb purification, continuous or multi-column chromatography is considered beneficial in many cases, as it, in addition to the factors mentioned above, also offers improved protein A resin capacity utilization. Key benefits of continuous chromatography are the fast processing times and reduction of holding times, which are critical for unstable molecules. There are, however, still challenges to overcome with higher complexity in hardware systems and some perceived regulatory uncertainties. Continuous chromatography is attracting a lot of interest in process development and clinical phases, but we can expect implementations in commercial manufacturing very soon. Nevertheless, there needs to be a thorough analysis for each project before determining if continuous or batch chromatography is the optimal choice (Fig 2).
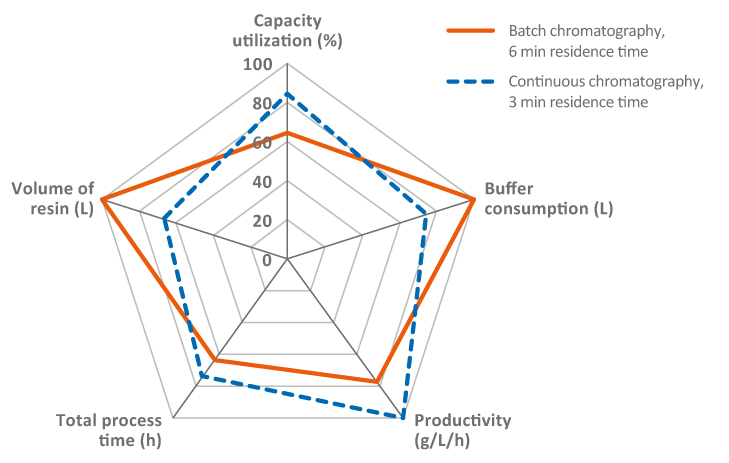
Fig 2. Relationships of parameters between batch and continuous Protein A chromatography step (MabSelect SuRe LX, 2000 L bioreactor feed at 5 g/L titer). Image illustrates how priorities and tradeoffs need to be optimized depending on desired outputs.
Fiber-based chromatography
Despite the evolution and effectiveness of Protein A chromatography resins, there are still challenges to overcome as we see industry going to smaller batch sizes and more multiproduct facilities. The purification process can be time-consuming, as one cycle typically takes 4 to 8 hours for industrial processing. If a biomanufacturer wants to run more than one cycle, the duration of the purification can take multiple days, resulting in process limitations and cost implications based on the facility setup. In addition, the resin must be cleaned and stored in a temperature-controlled cleanroom, leading to additional expenses. An alternative purification method currently under development is Fibro technology, which uses a novel proprietary structure to overcome the diffusional and flow limitations of packed bed chromatography purification systems.
With Protein A, target molecules carried in the bulk feed media must go through two stages of mass transfer before reaching the functional binding surface buried deep within the pores of a chromatography bead. The initial film diffusion, followed by diffusion into the porous structure, results in a flow-rate-limited operation, which presents a fundamental barrier to improving purification productivity. Instead, the structure of the fibers in the Fibro technology is highly open to facilitate mass transfer without diffusion, like membrane and monolith technologies, but it also yields a high surface area. This is a result of its manufacturing method, an additive process known as electrospinning. These unique features of Fibro allow it to obtain binding capacities of more than 30 mg/mL in seconds of residence time rather than minutes as with conventional bead chromatography. This enables purification cycles of around 5 minutes, as opposed to the traditional hours. The fast mAb purification allows much faster total process times. The fast purification process can alternatively be used to decrease the size and the cost of the purification media by cycling the Fibro unit up to 200 times in a single batch, thereby utilizing the full lifetime of the Fibro unit in a single batch and enabling a cost-effective true single-use chromatography (Fig 3).
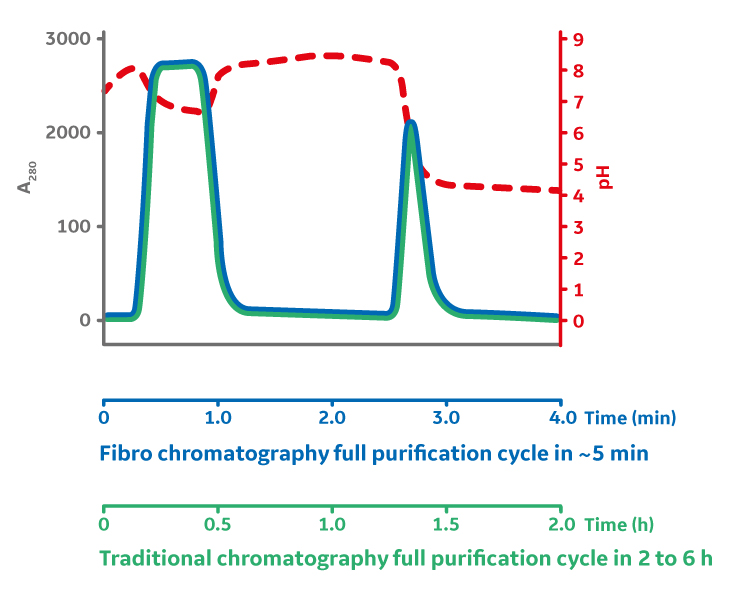
Fig 3. Fiber chromatography benefits include increased productivity (up to 400g/h/l), up to 50 times increased throughput for research and process development applications, and quick changeovers for flexible, highly productive manufacturing.
The industry has previously sought new technologies that could replace protein A chromatography. However, this is difficult, due to not only its effectiveness but also because protein A is nearly universally employed for mAb manufacturing. Instead, Fibro will offer a step-change in productivity, extending the use of protein A into the future of bioprocessing.
Fibro chromatography will have its main advantage in single-use equipment facilities, i.e., at small to medium processing scale (primarily up to 2000-liter bioreactor volume) and for multiproduct facilities where short turnover times are required or for applications where very fast mAb purification is desired.
Determining the best fit for your facility will be based on how many products you produce at once, the region your company operates in, and where you are in the clinical space. Once these factors are considered, partner with a supplier that can offer a broad range of solutions to address your specific needs and that demonstrates an ability to develop new technologies that keep pace with the evolution of today’s industry.
- G. Jagschies,Managing manufacturing economics, in: H. Levine, G. Jagschies (Eds.), The Development of Therapeutic Monoclonal Antibodies, Bioprocess Technology Consultants and Cytiva, Acton, MA/Uppsala, 2010, pp. 307–332.
- Cytiva (2018).Process-economy simulation of mAb capture step with MabSelect PrismA protein A chromatography resin. Retrieved from https://cdn.cytivalifesciences.com/api/public/content/digi-28219-pdf
Discover more in the Process Efficiency series: