Process intensification strategies aiming to maximize facility utilization and increase output for fed-batch processes can potentially increase productivity and reduce cost in biomanufacturing using mammalian cell culture. We will compare conventional seed train methods to the use of high-cell density (HCD) cryobags. We will show that high-density cryobags can be used to seed a bioreactor at a 500-fold higher seed density, reducing the total process time by up to 9 days.
Introduction
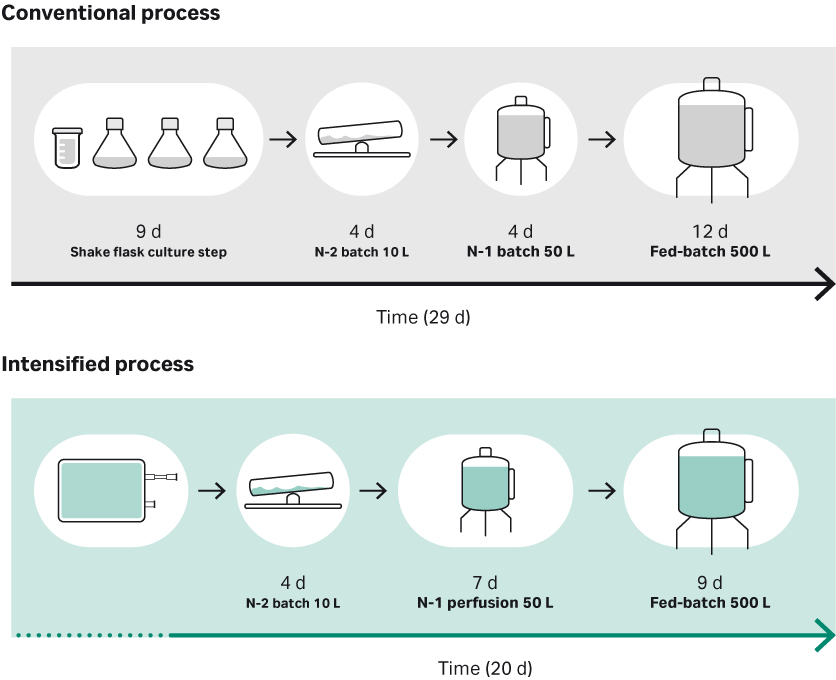
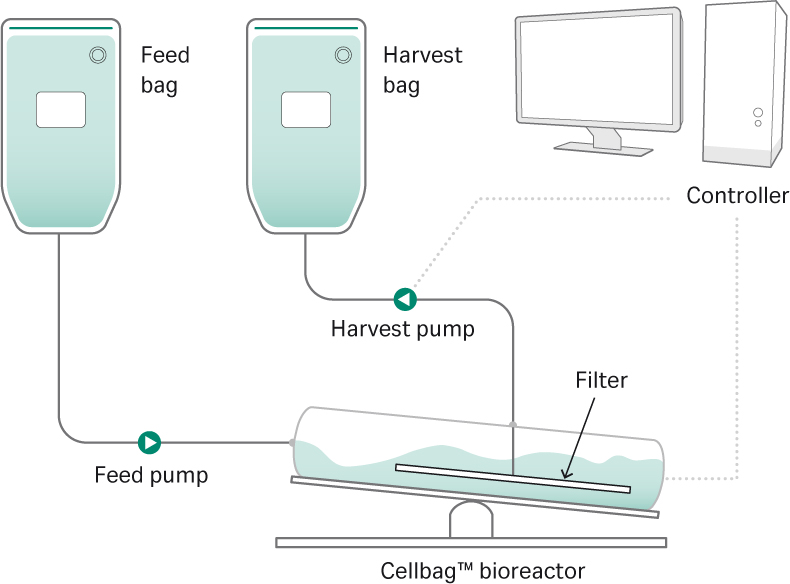
Traditionally, a seed train starts with a small vial of cells that is thawed and expanded to hundreds or even thousands of liters for single-use bioreactors, taking weeks to complete. Such a procedure is time-consuming and labor intensive, and each manual interaction constitutes a risk of contamination of the culture. The alternative is to use HCD vials or cryobags that reduce the cell expansion process by days or even weeks, utilizing manufacturing capability more efficiently. The use of cryobags in seed culture expansion allows for a one-step process, from cryobag to seed bioreactor, omitting the need for intermittent seed culture steps in shake flasks and associated labor (Fig 1). Additionally, cryobags may remove the need for expensive clean-room facilities and due to their single-use compatibility will help reduce start-up time.
To form an intensified seed process including a high-density cell bank, one cryobag can be thawed and used to directly inoculate a N-2 or even a N-1 seed bioreactor, depending on the number of cryopreserved cells. As an example, an HCD cryobag can be used to inoculate a ReadyToProcess WAVE™ 25 bioreactor, where culturing continues in batch mode until a culture time of four days is reached, after which the culture can be transferred to a N-1 step and a 50 L XDR-50 bioreactor operated in perfusion mode. The described method will generate enough cells to seed a 500 L production bioreactor at high cell concentration in only 11 days.
Fig 1. Overview of conventional versus the intensified high-density seed process evaluated in the study.
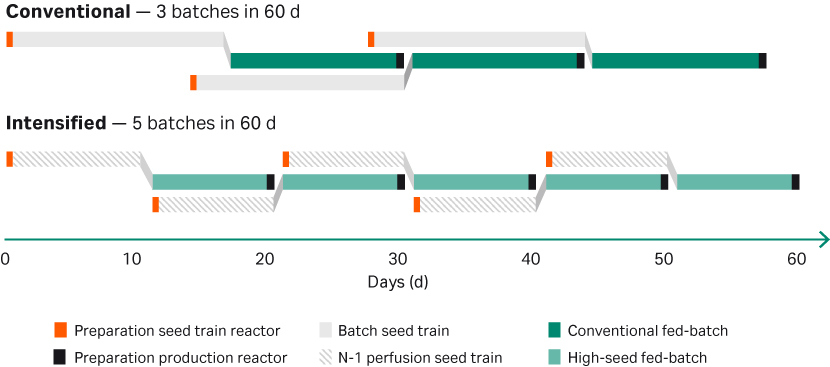
Five campaigns can be fit within a 60-day time frame utilizing the potential of an intensified seed train, including an initial cryobag thawed directly into the N-2 seed bioreactor, followed by a N-1 perfusion seed bioreactor, and a final production bioreactor. The final production process can be intensified by seeding at a higher cell density compared to a conventional seeding cell density reducing the production phase by 3 to 5 days (Fig 2).
Fig 2. Potential capacity increase achieved by introducing HCD cryobags in the seed train.
Results
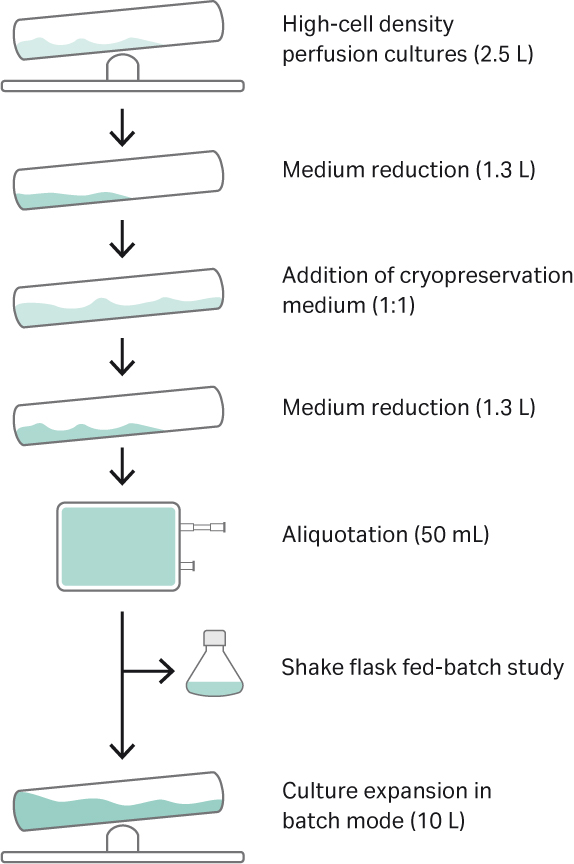
The experiment was performed in three parts. The first part was focused on the generation of the HCD cryobags (Fig 3). In the second part, we thawed HCD cryobags directly into the WAVE™ 25 bioreactor instead of shake flasks. In the third and final part, one HCD cryobag was thawed and validated in a fed-batch study in shake flasks. The impact of HCD stock preparation on cell recovery after thaw, cell growth, and IgG titers when compared to control cultures was investigated. The control cultures were in LCD vials that were thawed conventionally in a water bath, washed free from DMSO, and maintained in shake flasks.
Fig 3. Overall experimental setup for generating and validation of the cryobags.
Generation of HCD cryobags and cryovials
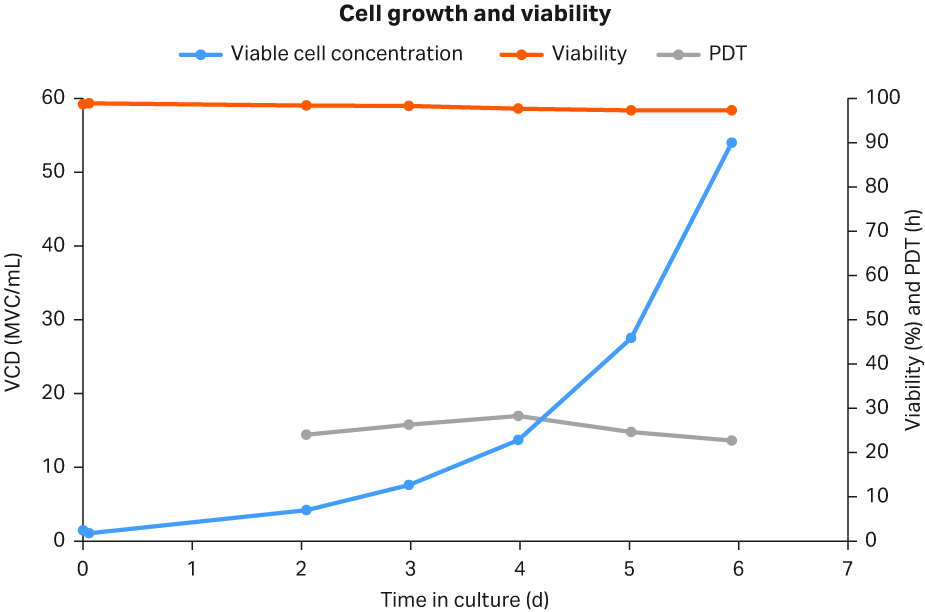
To generate cryobags, cells cultured in shake flasks were used to inoculate the Cellbag™ bioreactor at 1 MVC/mL and 2.5 L on day 0. The cells were grown in batch mode until day 2, then they were kept in perfusion mode from day 2 to 6. The cells grew at an exponential growth rate and peaked at 54 MVC/mL on day 6, with viability remaining high throughout at no less than 97.3% on day 6 (Fig 4). The population doubling time (PDT) was well within the acceptance criteria of 40 h at time of harvest reached on day 6.
Fig 4. Cell growth, cell viability, and PDT of cells grown in WAVE™ 25 bioreactor for the purpose of generating HCD cryobags.
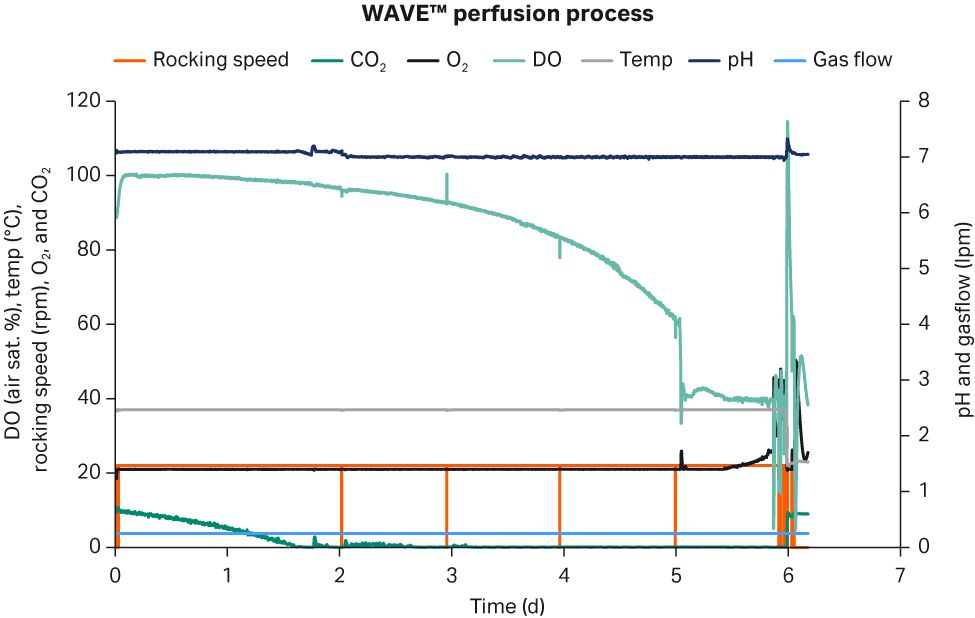
All critical process parameters such as DO, pH, and temperature were stable throughout the perfusion culture (Fig 5).
Fig 5. Process parameters during perfusion process in WAVE™ bioreactor during cryobag generation.
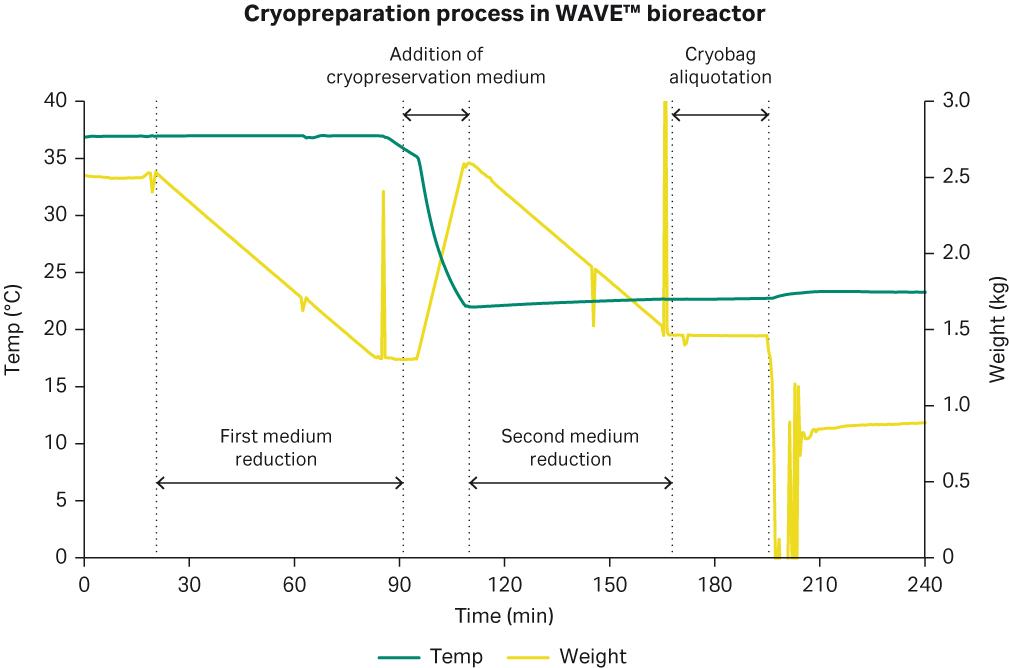
At 6 days and a generation number of 26, the cryobag preparation phase was started by stopping the harvest pump, allowing the weight to reduce from 2.5 to 1.3 kg in approximately 1 h (Fig 6) to reach a VCD of approximately 100 MVC/mL. Thereafter, temperature was stopped, and 1.3 L of 15% DMSO plus ActiPro™ cryopreservation media was added to the Cellbag™. The weight was increased to 2.5 kg, mixed for 10 min and once again, cells were concentrated for 1 h to their final cell concentration of 113 MVC/mL and 50 mg of cell suspension was dispensed into each of the four cryobags. In addition, 50 mg of cell suspension was added to an additional bottle for cryopreservation in 4.5 mL vials. 4 mL of the cell suspension in the additional bottle was dispensed into each of the 12 cryovials.
Fig 6. Cryopreparation process in WAVE™ bioreactor.
Cell recovery after thaw and direct seeding
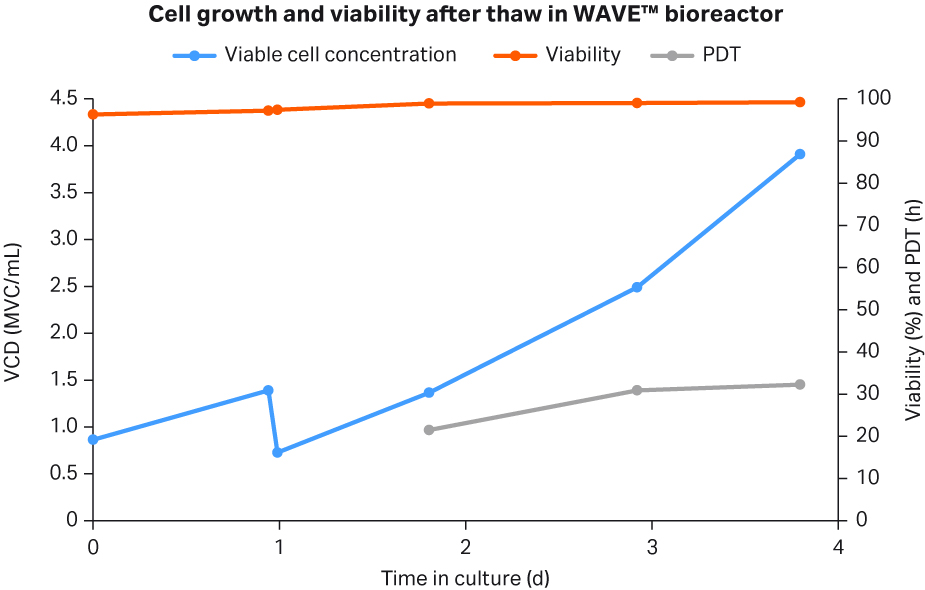
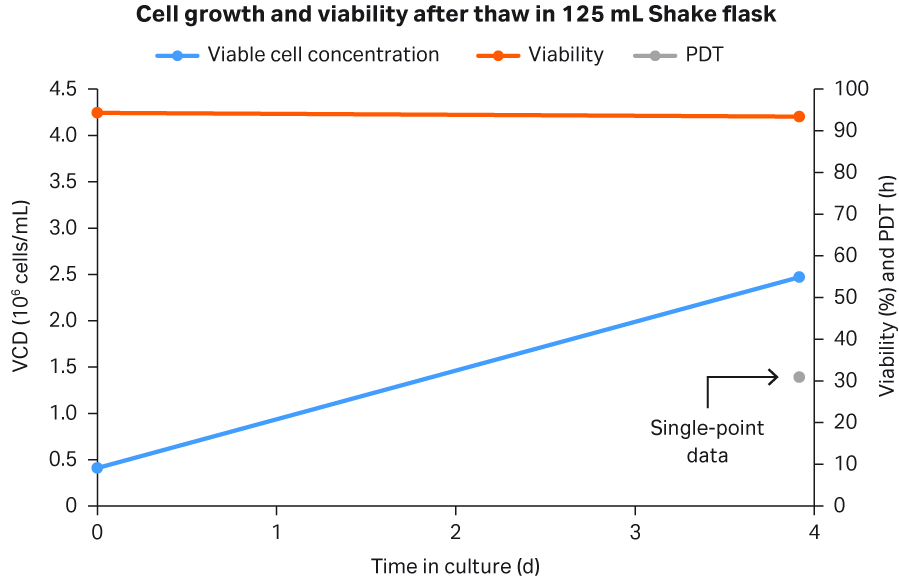
To verify that the cells recovered from cryostorage and that direct inoculation into a seed bioreactor without significant stalling was possible, one HCD cryobag was thawed and seeded directly into WAVE™ bioreactor at 5 L. Cell viability (96.3%) was similar after thaw at day 0 (Fig 7) to the control culture (94.3%) (Fig 8). In addition, the PDT averages at 28.2 h was similar to the PDT of 30.9 h for the control culture (single point data in Fig 8). Control cultures used for comparison to the intensified process were standard low-cell density (LCD) cryovials thawed conventionally, washed free from DMSO, and transferred into shake flasks.
Fig 7. Cell growth, viability, and PDT for WAVE™ bioreactor seeded with HCD cryobag.
Fig 8. Cell growth, viability, and PDT for 125 mL shake flask seeded with LCD cryovial.
Fed-batch cultures
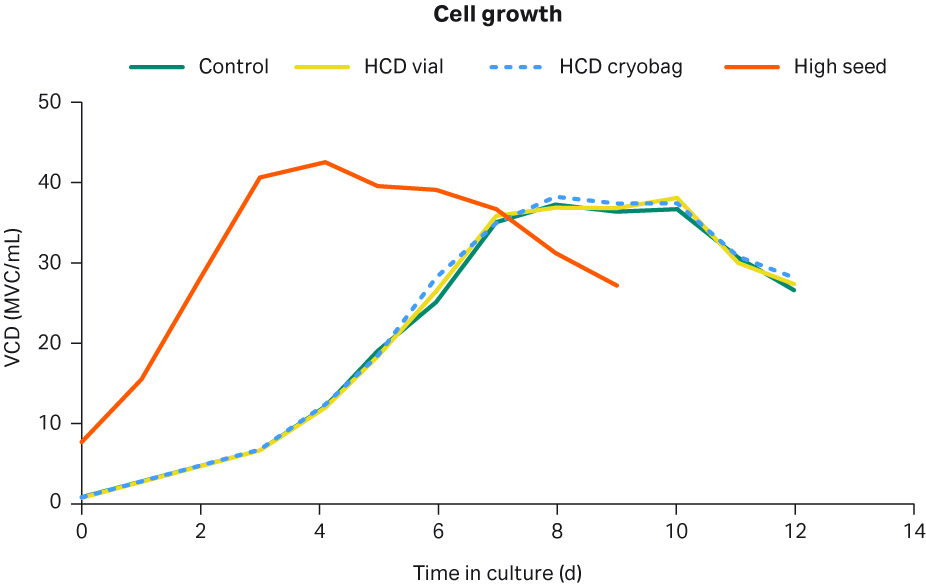
To validate that the cell growth and IgG productivity in the production phase were not affected by the use of HCD cryobags or HCD vials, cells where thawed and maintained in shake flasks for nine days to simulate an intensified seed train process and then seeded for fed-batch cultures in shake flasks. A set of eight shake flasks or four duplicates were run as fed-batch cultures, as described in the materials and methods section at the end of this article, to validate the HCD cryovial and cryobag cells against LCD control cell cultures. One set of duplicate shake flasks were seeded with a high cell density of 8 MVC/mL to test if the production phase could be further shortened with maintained cell growth and IgG productivity compared to the conventionally seeded cultures. Figure 9 shows the cell growth as an average of the each of the duplicate cell cultures.
The growth of the high seed cultures shows that the growth rate after seeding was maintained with no signs of a lag phase. The high seed growth rate was similar to the conventional process, with a peak VCD of 43.5 MVC/mL. However, the high seed culture reached its peak approximately four days earlier than the conventional processes. The growth curves of the conventional processes were similar both in terms of growth rate and peak VCD. HCD vial cells reached 36.9 MVC/mL, HCD cryobag cells reached 38.2 MVC/mL, while the control cells reached 37.3 MVC/mL.
Fig 9. Cell growth profiles for the control cells (green), HCD vial cells (yellow), HCD cryobag conventionally seeded (dashed blue), and the high-seed fed-batch process (orange) in shake flasks.
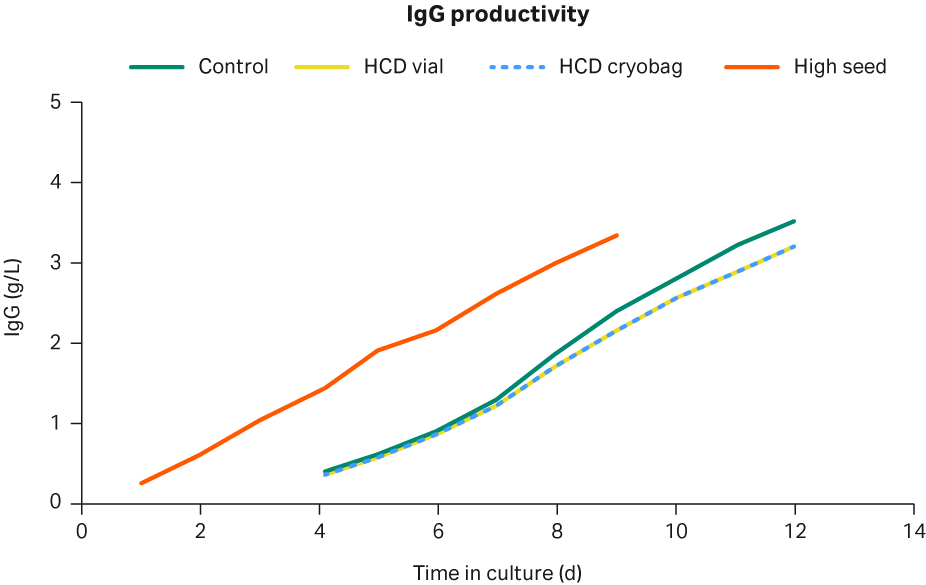
Figure 10 shows the mAb titers for the different cultures. The control cells reached a final mAb titer of 3.5 g/L, while the HCD vial and HCD cryobags reached a similar final mAb titer of 3.2 g/L. The high seed culture reached a final mAb titer of 3.3 g/L three days earlier than the conventional processes.
Fig 10. IgG profiles for the control cells (green), HCD vial cells (yellow), HCD cryobag conventionally seeded (dashed blue), and the high-seed fed-batch process (orange) in shake flasks.
The results show that both cell growth and product titers for all cell cultures reached comparable levels. The high seed process reduced the production duration by three days. There were no significant differences between control cells and HCD cryopreserved cells in terms of growth and productivity.
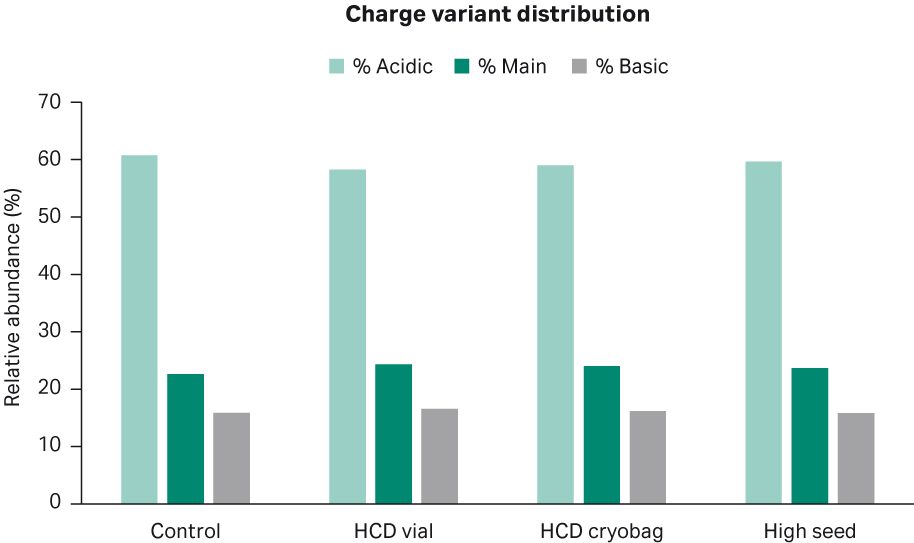
A sample from each shake flask culture was removed on the day of harvest for further analysis of product quality attributes such as glycosylation, charge variant distribution, and aggregation levels. The charge variant distribution between the different shake flask cultures had comparable profiles with approximately 60% acidic variants, 24% main variants, and 16% basic variants, respectively (Fig 11).
Fig 11. Average distribution of different charge variants for the duplicate shake flask cultures.
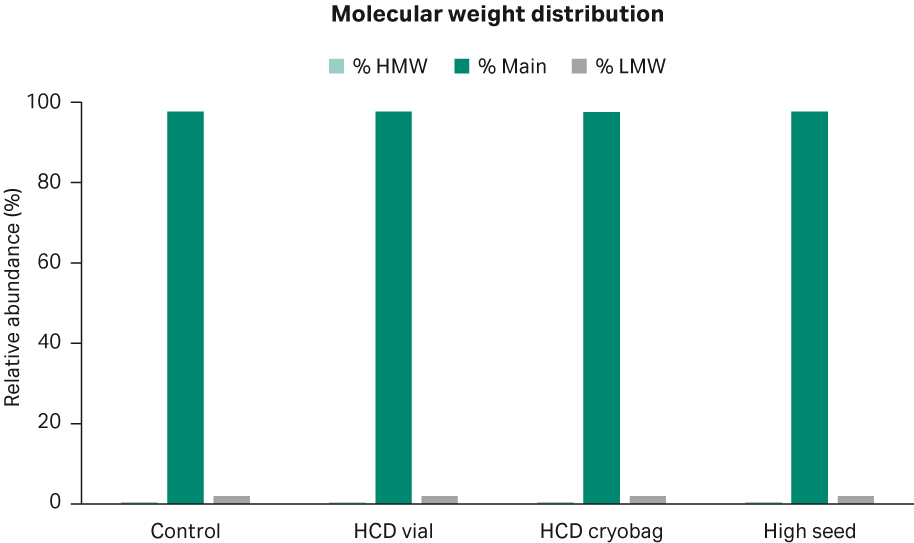
0.5% of high-molecular weight (HMW) particles and 2% of low-molecular weight (LMW) particles were observed in all shake flask cultures, respectively (Fig 12).
Fig 12. Average distribution of high-molecular, low-molecular, and main weight molecules from duplicate shake flask cultures.
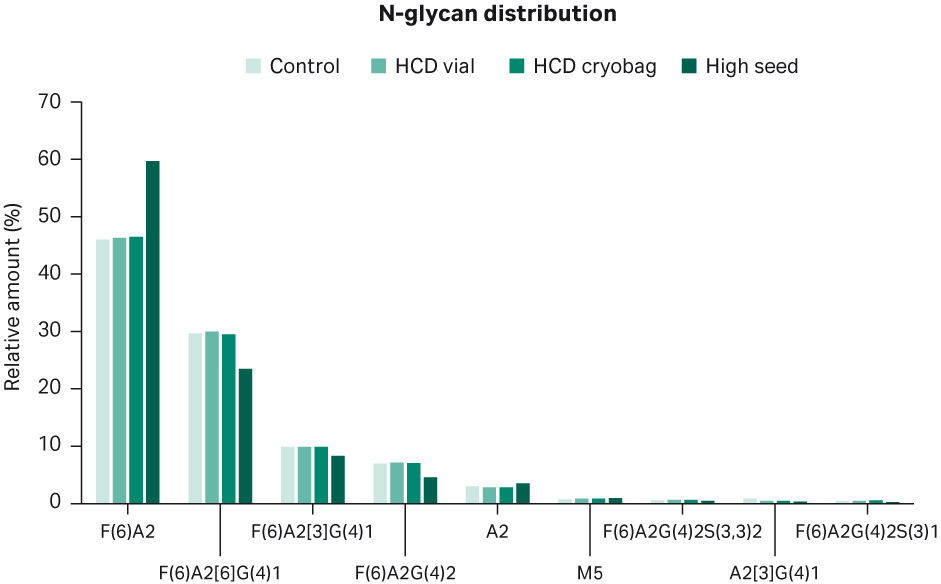
The relative distribution of N-glycans on the product between the different shake flask cultures is shown in Figure 13. Control HCD vial and HCD cryobag shake flask cultures had a relatively comparable distribution of N-glycans, with around 46% F(6)A2, 30% F(6)A2[6]G(4)1, 10% F(6)A[3]G(4)2, 7% F(6)A2G(4)2, 2% A2, and the remaining below 1% each. High seed shake flask cultures had a different distribution of variants F(6)A2 and F(6)A2[6]G(4)1, being 60% and 23% on average, respectively.
Fig 13. Average distribution of N-glycans for duplicate shake flask cultures.
Conclusions
We have shown that HCD banks using CHO cells in cryobags can be created in a closed manner using the ReadyToProcess WAVE™ bioreactor in perfusion mode in only six days. The described method generated enough cells to perform a direct seed of N-2 seed and 5 L bioreactors with maintained growth and high cell viability. With the achieved total cell concentration in HCD cryobags, the seed train duration could be shortened by nine days compared to traditional LCD vials without affecting the growth or titer profile of the final product.
In addition, the use of cryobags for seed culture expansion allowed a one-step process, from cryobag to N-2 bioreactor, omitting the need for intermittent seed culture steps in shake flasks. This intensified procedure allows for greater productivity in comparison to conventional seed train processes by reducing the time, labor, and materials needed, while also reducing the risk of contamination of the culture.
Generation of HCD cryobags and cryovials
First, mAb8 cells from LCD control vials were thawed in a shake flask and expanded for 11 days. They were then seeded into a WAVE™ 25 bioreactor and a 10 L WAVE™ bioreactor perfusion Cellbag™ (Table 1). After seeding and two days in batch mode, the perfusion process was initiated at a CSPR of 140 pL/cell/d with ActiPro™ cell culture media (Fig 14).
Table 1. Culture conditions for perfusion process in WAVE™ 25 bioreactor
| Parameter | Setting |
| Culture working volume | 2.5 L in 10 L Cellbag™ |
| Rocking rate | 22 rpm |
| Temperature setpoint | 37°C |
| Target inoculation cell concentration | 1 MVC/mL |
| pH setpoint | 7.0 |
| DO setpoint | 40% air sat. |
| CSPR | 140 pL/cell/day. |
| Target viable cell concentration | 50 MVC/mL |
| Target viability | 95% |
| Culture duration | 6 days |
| Perfusion cell culture media | ActiPro™ |
Fig 14. Perfusion setup for ReadyToProcess WAVE™ 25 bioreactor.
After four days in perfusion mode, cells were prepared for cryopreservation.
Four cryobags were inter-connected with a C-flex® 5-way jumper. The Cellbag™ was tilted and the jumper welded to it. One cryobag at a time was placed on a scale before each filling. Once filled the cryobag was clamped and replaced with a new cryobag.
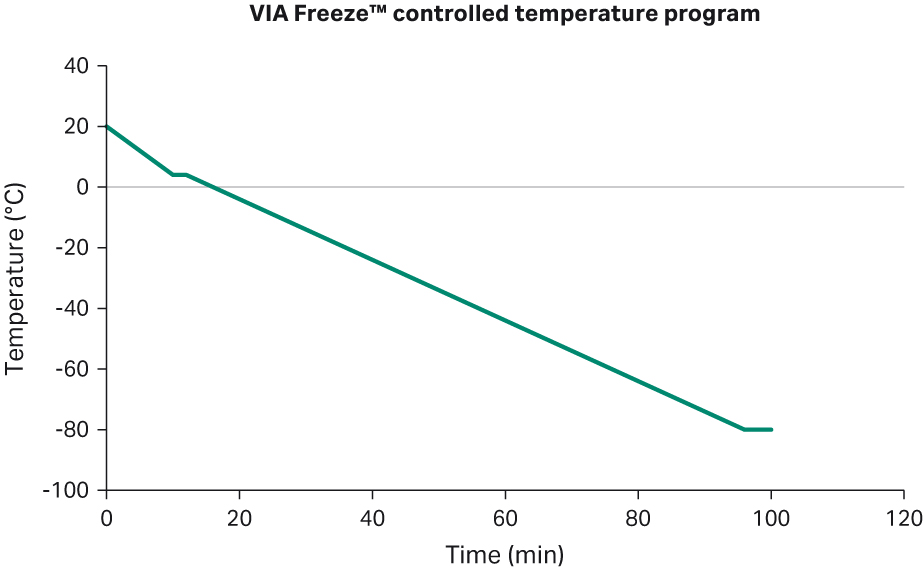
When all the cryobags were filled, the jumper connected to the Cellbag™ was sealed off and the cryobags were removed. Before cryopreservation, the jumper was sealed off from each cryobag and placed inside cassettes. These cassettes were then placed inside a rack within the VIA Freeze™ Duo and the cryoprotocol was initiated (Fig 15). The cryovials were put into an iso-propanol cryopreservation container and stored in the -80°C freezer.
Fig 15. Cryotemp profile in VIA Freeze™ Duo.
Generation of HCD cryobags and cryovials
A cryobag was collected from the -80°C freezer and placed inside the VIA Thaw™ L1000 with settings of 50 mL, -70°C, and 10 min. One of the openings on the cryobag was peeled off and a Clave™ spike connector with female luer needle was pushed into the opening. To the Clave™ spike connector, a male luer connecter with C-flex® tubing was connected. The cryobag was then placed next to the Cellbag™ and welded onto one of the 1/8" tubing. The content of the bag was pumped into the WAVE Cellbag™ using a Watson-Marlow 120U pump at 35 rpm and the working volume was increased to 5 L (Table 2). The control cultures were thawed in a water bath then transferred to centrifugal tubes with basal media. They were then centrifuged at 120 × g and resuspended in the same media. The control cultures were then seeded into shake flasks and maintained at the same conditions.
Table 2. Culture conditions for batch process in WAVE™ 25 bioreactor
| Parameter | Setting |
| Culture working volume | 5 L and expanded to 10 L on day 1 in 20 L Cellbag™ |
| Rocking rate | 22 rpm |
| Temperature setpoint | 37°C |
| Target inoculation cell concentration | 0.8 to 1 MVC/mL |
| pH control | 7.5% CO2 gas mix |
| DO control | Oxygen enriched gas mix on demand. |
| DO setpoint | 40% air sat. |
| Target viable cell concentration | 3.5 to 5 MVC/mL |
| Target viability | 95% |
| Culture duration | 4 days |
| Cell culture media | ActiPro™ |
After 30 min a sample was taken from the Cellbag™ and pH, cell concentration, and viability were measured. After one day and sampling, the volume was increased to 10 L. After an additional three days of culture, the culture was finalized.
Fed-batch culture in shake flasks
A second HCD cryobag was thawed using VIA Thaw™, while one HCD cryovial and one control vial were thawed in a water bath. To simulate a conventional seed train process, control cells were maintained for 23 days while HCD cells were maintained in a shake incubator for nine days, measured, and seeded according to the following setup:
- Flasks 1 and 2 were seeded from the control culture at 0.8 MVC/mL in 40 mL of ActiPro™ cell culture media.
- Flasks 3 and 4 were seeded from HCD vial culture at 0.8 MVC/mL in 40 mL of ActiPro™ cell culture media.
- Flasks 5 and 6 were seeded from HCD cryobag culture at 0.8 MVC/mL in 40 mL of ActiPro™ cell culture media.
- Flasks 7 and 8 were seeded from the HCD cryobag culture at 8 MVC/mL in 40 mL ActiPro™ plus 2.5% HyClone™ Cell Boost™ 7a supplement and 0.25% Feed 7b cell culture media.
After 30 min of incubation, cell concentration and viability were measured for all flasks. Feeding started on day 1 for Flasks 7 and 8, and on day 3 for Flasks 1 through 6.