Biomanufacturing is under increased pressure to adapt to market demands, such as reducing costs and bringing life-saving therapies to the people who need it faster. One method of achieving these goals is through closed connected single-use processing. Some of the advantages of closed processing include shorter processing time, fewer manual interactions throughout the process, decreased capital expenditures (CAPEX), reduced resources for cleaning and validation, and diminished footprints for qualified manufacturing space.
While closed connected processing offers advantages, it also requires new approaches with respect to process development, planning, and execution. Evaluation of new process methodologies is both time and resource consuming and can often be delayed or truncated. We performed an evaluation of biomanufacturing technologies, which can potentially improve efficiencies, and offer our findings as additional data for consideration when adopting these methods. The purpose of this discussion is to summarize our recommendations from our execution of a closed and connected mAb process from design considerations to scale-up, including the small-scale modeling that directed our process at manufacturing scale.
Designing a closed connected process
As individual unit operations are becoming more efficient, focus has shifted to consider efficiencies across an entire manufacturing process. One way to achieve greater efficiency is through implementation of closed and connected processing. It cannot be overstated that successfully performing a closed connected process is linked to the amount of effort exerted in the preparation phase and particularly the specific design considerations. In comparison to traditional bioprocessing, the focus when designing, executing and implementing a closed connected single-use process should be on the planning and preparation phases, as described in Process considerations for closed connected processing. Proactive planning will help to anticipate and mitigate risks and ensure a seamless final execution of the process.
Depending on scale and equipment vendors, there are many components to manage in one single-use production batch. Each connection must be verified for fit and function, with attention to reducing the total number of parts by examining common denominators for potential consolidation of single-use expendables.
For connected processes it is important to consider the length of the tubing connecting the different unit operations carefully. It is beneficial from a contamination risk viewpoint to design the tubing length as short as possible, to make the setup easy to survey, facilitate the transfer of the liquid and minimize dead legs and losses in the tubing. This type of setup needs to be carefully planned and knowledge of the equipment used such as its appearance, size, contexture, etc., is necessary.
One lesson learned from our upstream process was to visualize the connections in the lab and map out where the tubing will go and where the connections will be performed. Since operators are not able to disconnect and reconnect tubing to the process line on a closed, connected process, the flexibility for process changes is limited. To increase the flexibility and to create a setup that is more amenable to ad hoc adjustments, addition of Y-manifolds or T-manifolds at critical points may be helpful should an extra filter or buffer bag connection be needed.
It was also important to not only map out the process on paper but also set up the entire process for a dry-run and realistically look for issues, such as access restrictions and tubing networks or crossings, which impose safety or contamination risks. We identified several such issues in our design that were remedied because we took the added step to set up a simulation of the process. At this point, it is prudent to create a bill of materials for all single-use components and identify lead times and develop security of supply plans. Our project plan included extra components, since this was a new process. This may be reduced to one extra set once the process is defined and both supply and execution runs successfully concluded.
Understanding the mass balance is also critical in a connected process to avoid mismatches in unit operations sizing, which could cause detrimental breaches or delays. A connected process will not be as forgiving as a disconnected process if the process flows, titers, capacities, and yields are not well understood upfront.
For a well-known process, adapting column sizes and other consumables and equipment to the current scale is overall straight forward. However, during the development phase, where process parameters and outcomes are more uncertain, it is important to predict variations and consider safety margins in the mass balance calculations. For example, the size of the PrismA column was dimensioned with some overcapacity and could therefore handle all produced material even if the titer became higher than estimated. For the polishing step, using Capto S Impact, on the other hand, the column was undersized. To avoid wasting material connecting additional outlet ports enables more cycles to be performed as needed without breaking the closed setup.
We also calculated the amount of buffer required based on scale and estimated product yield. The in-line conditioning (IC) skid was used for buffer preparation from stock solutions. The IC system was set up as a buffer kitchen with premade stock solutions connected to the inlets. IC helps to reduce volumes and hold up storage, especially if it used as a chromatography skid delivering buffer directly to the column.
To ensure that buffers and media were not depleted during the process approximately 20 to 30% extra buffer was prepared. In our 50 L perfusion bioreactor, we estimate we will need approximately 800 L of cell culture media but made 1000 L to account for the 25% safety factor. One recommendation is to use the same stock solutions for as many buffers as possible. Additionally, during buffer preparation, we recommend to make buffers based on the same stock solutions sequentially to minimize the priming needs.
Process optimization studies
Small-scale model to increase yield in perfusion cell culture
Another important element of a successful closed connected process is identifying and evaluating the critical process parameters before performing the large-scale production. For example, hold time studies for product and buffer/media compositions and volumes, product intermediate volumes, and process settings should be established in the planning phase. Small-scale studies can lead to a higher likelihood of a successful scale-up, by helping to inform risk mitigation strategies in a controlled proactive manner instead of as a result of large-scale deviation. While this seems to be common sense, a challenge here is to create a good small-scale replica of the larger scale process. Smaller equipment as well as process dynamics may not be the same and an assessment of the applicability of data at large-scale is often difficult.
To characterize the process parameters that could increase yield in a large-scale perfusion process we developed a small-scale perfusion model using ReadyToProcess WAVE 25 Rocker bioreactor and WAVE Cellbag 50 L. In our model, the cell line and media used was consistent with the larger scale process, so we felt that the small-scale model was an acceptable reference for performance at the large scale.
The effect of temperature was first tested in TPP® TubeSpin® Bioreactor tubes of 50 mL with Chinese Hamster Ovary (CHO) cells in a humified shake incubator. This tube-spin setup investigated the possible effect of a temperature shift from 37°C to 31°C and if the time when this temperature shift was introduced had any effect on cell culture productivity. The results showed that the temperature shift did not improve productivity of our cell line and the perfusion culture in ReadyToProcess WAVE 25 was therefore run at 37°C.
We also examined the optimum viable cell density and its association with productivity in the TubeSpin experiments. Data from the experiment supported that operating at a higher viable cell density increased the total product yield by 40%. This information was used to set the process parameters for the larger scale connected process. We successfully scaled-up to a 50 L WAVE Cellbag bioreactor which was run for a total of 12 days. Daily harvest samples were collected at controlled state of 70 MVC/mL, with an average volume productivity of 0.85 g/L/d for 3 days.
Downstream pre-studies
A standard MabSelect PrismA method including low pH viral inactivation was evaluated at lab-scale using HiScreen prepacked columns and HiTrap PrismA Fibro units. Running conditions, dynamic binding capacities, product stability, etc., were studied to understand the basis for scale-up in all separate unit operations.
The Capto S ImpAct step was evaluated at pH 5.0 since this was considered a favorable condition for the mAb. After optimization, a step elution using 50 mM NaAcetate, 200 mM NaCl was shown to give a robust separation with a concentrated elution peak and high reduction of host cell proteins (HCP) and residual protein A. Aggregate levels were reduced to below 0.7%. Subsequently, a study with different column loads was done to understand if load amount affects the separation and elution profile. The performance of the step was shown to be independent of column load which was useful information before scale-up.
For the final polishing step, using a Q Membrane Adsorber in flow through mode, an initially study at pH 7.5 was done. We found that there was a high reduction of HCPs, and DNA levels where generally below detection limits for the Q Membrane Adsorber in flow through mode. However, stability issues with the antibody were seen and high molecular weight (HMW) levels did increase significantly at this pH. After additional experiments, it was found that pH 6.0 was the optimal condition for this step.
Finally, a down-scaled model of the planned large-scale process was established, and a complete process run was done to confirm the process as a whole. Total yield over the three chromatography steps including viral inactivation in the final process run was 88%.
Process run
Large-scale connected perfusion cell culture
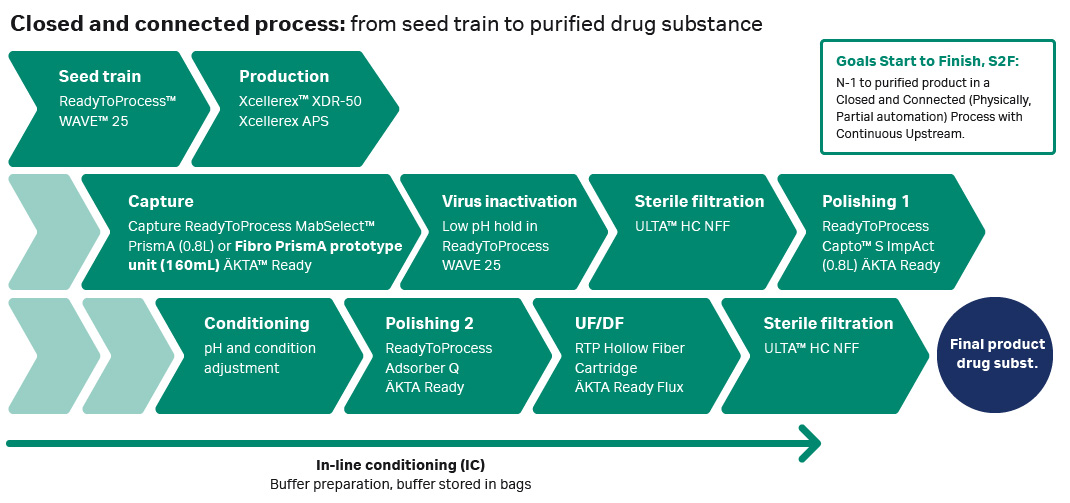
Our upstream process consisted of an Xcellerex XDR50 bioreactor connected to an Xcellerex automatic perfusion system (APS), which performed continuous clarification of the cell harvest and collection of the product. The product was then collected in an Xcellerex XDM 200 hold tank for subsequent downstream processing (Fig 1).
Perfusion processes enable continuous operation over an extended period of time, by constantly providing fresh nutrients for the cells and simultaneously removing waste products, as compared to batch and fed batch process. The goal of perfusion is to improve efficiencies through increased yield, faster processing, optimal use of materials, smaller overall footprint, and consistent and improved product quality.
Fig 1. Process set up from vial to 50 L perfusion cell culture process to dual downstream processing trains.
The Xcellerex APS perfusion system was a key component in this connected upstream process. It utilizes hollow fiber microfilters as a cell retention method. The tangential flow filtration (TFF) based system consists of a low shear pump to recirculate process fluid through hollow fiber filters and back to the bioreactor. Pumps for perfusion media feed addition, permeate harvest, and cell bleed are controlled by feed-back controllers. Single-use pressure sensors enable continuous online monitoring of transmembrane pressure (TMP) and pressure differential (delta P) to optimize filter performance. The filter did not foul or clog during the entire run and only a single filter was used throughout the run. This is critical, since it saves time and money and reduces risk of disconnecting the system to replace the filter. The automation included in the APS simplified the daily work with automatic changes to the perfusion and bleed rates. As with all automation it also introduces complexity so that proper training of the operators is crucial.
In conclusion, we were able to achieve a high cell density of 70 MVC/mL, with a cell viability of greater than 90% for 10 days. In addition, the cell harvest delivered a daily average IgG titer of 0.72 g/L to the harvest hold bag and maintained a cell-specific perfusion rate (CSPR) of 20 pL/cell/day.
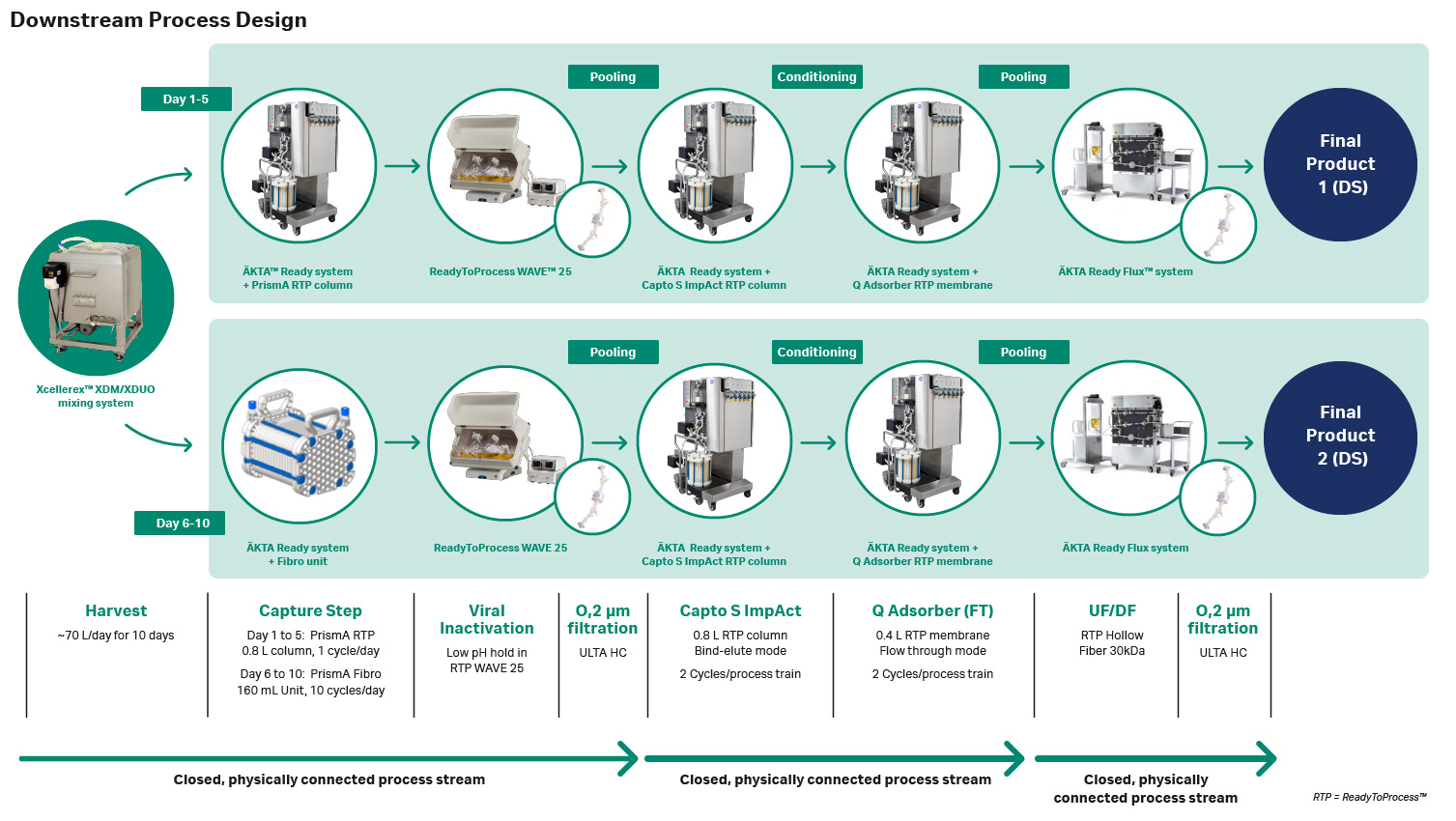
Large-scale closed downstream purification process
Downstream purification is typically performed using resin-based chromatography methods. New, fiber-based chromatography methods, which offer improved efficiency, have recently been introduced. In this study, the purification process was divided into two separate processing setups, as shown in Figure 2, to evaluate the performance of both resin-based chromatography and fiber-based chromatography. The harvest material from days one through five were purified using ReadyToProcess MabSelect PrismA columns for the capture step. While the materials from days six through ten were purified using a Fibro PrismA unit, which utilizes a fiber-based technology that enables purification cycles within minutes for the capture step.
The two polishing steps were connected physically through the ÄKTA systems with a hold bag between for conditioning purposes. Connecting two steps in a closed way, as we did with the Capto S ImpAct to Q Adsorber has a number of advantages related to efficiency and intensification. As the product can be conditioned instantly and processed directly without any storage, handling of the product intermediate is minimized, and the risk of contamination decreased. In addition, no sterile filtration or storage is needed between the polishing steps when the product is processed directly without a hold. There may also be an advantage from a product stability perspective to connect the steps and continue the process directly. In our lab-scale experiments, we found that the eluate from the first polishing step was unstable during storage and therefore it was necessary to process it directly to keep the product in a more suitable buffer before storage.
At the pooling points in the process, see Figure 2, shorter intermediate holds were included for practical reasons. Without a fully automated process line this is needed to be able to disassemble already used equipment and set up equipment for the coming unit operations. For scheduling purposes, everyday process holds are also needed to accommodate for regular working hours and weekends. To secure product quality during process holds it is crucial to evaluate the stability of the intermediates at the relevant conditions.
Fig 2. Large-scale downstream process overview.
During the large-scale downstream process run, there were two findings that were not seen in the small-scale studies. First, during the viral inactivation step a large increase in aggregate formation was observed, which varied from sub-batch to sub-batch. This increase in aggregate formation was likely the result of the larger volume and localized spots of high acid concentration when adding acid. This finding indicates that further optimization is needed to perform the viral inactivation step at scale-up. The rocking rate, the type of acid, its concentration, and the addition rate of the acid need to be further evaluated to ensure improved performance of this step. Second, the yield for the viral inactivation step also varied for the process train using the MabSelect PrismA column. Volume losses was larger in the first two sub-batches and lower in the final three sub-batches. The larger volume losses in the first two batches were likely the result of using a 0.2 µm filter with larger surface area for the filtration step.
Both process setups achieved a high-quality final product in terms of the critical quality attributes. In addition, bioburden and endotoxin levels were monitored throughout the process to demonstrate the aseptic status of the setup. No bioburden was detected in the majority of the analyzed intermediates, and repeat testing found no contamination and endotoxin levels were below detection limit for all samples collected downstream of the harvest tank. The main difference between the two processes was the processing time. The process time for the ReadyToProcess MabSelect PrismA runs were approximately 7.7 h/sub-batch. While the process time for the Fibro PrismA runs were approximately 3.0 h/sub-batch, which is substantial process time savings.
Both the Fibro unit, as well as the column set-up, were suitable for processing perfusion material coming direct from the Xcellerex APS without additional filtering needed. No clogging or pressure increase was observed for either the column or the Fibro unit during their five days of use. When scaling up Fibro processes, one important point to consider is that large scale equipment is normally designed for use with columns. Fibro units are often < 10-fold smaller than the corresponding column, but the Fibro flowrates are higher. This needs to be accounted for when setting the peak collection criteria, since the dead volume in the flow path of the large-scale equipment will have a larger impact for the smaller units compared to columns. Evaluating a few cycles at scale will ensure an optimized collection.
The rapid cycling Fibro chromatography also gave productivity gains due to its very fast mass transfer, with cycle times in minutes rather than hours. This proved very useful in this set-up. Halfway through our process we started getting more material out of the bioreactor than expected. And even if this is a good thing, it can lead to downstream challenges in a connected process like this. In our case, however, due to the very quick cycle times with the Fibro technology, we could easily go from the planned 7 cycles a day up to 10 cycles, allowing us to process all the material from the bioreactor in one working day. We needed less than an hour to perform these additional cycles and didn’t have to waste any of the bioreactor output.
The process for the UF/DF step was run automatically using the ÄKTA ready flux with a single-use flow path and UNICORN method. This set up produced a consistently high yield across batches while at the same time making the process easy and convenient to operate. All process buffers and solutions could be connected before starting the method and the programed method went through the different phases in the process step-by-step and adjusted TMP automatically as the flux through the filter changed. The operator also had a good overview of the process, since they could see the current open flow path on the screen and monitor retentate weight and permeate flow continuously.
Discussion
When focusing on a closed and connected process from the N-1 step to the final filtration step, the planning phase becomes even more crucial compared to standard bioprocessing. However, when all is said and done, communication between the involved teams is the true key to success. To achieve this, we worked close together as one team, in an adapted agile way, not as one upstream and one downstream team. Regular meetings with the complete team from twice a week up to daily, during the intense execution period, were important. Dependencies over all disciplines were sorted out in short scrum meetings with full status visualization on the scrum board, making it easy to track progress and identify potential risks. It also facilitated open and direct communication, enabling flexibility within the whole team. A major impact on success was to clearly state the goals and get all team members committed to that, as well as to the agile way of working and transparent communication.
An important activity for the team throughout the whole project was to identify new risks and keep mitigating them on a regular basis. This set way of working for the team made the task of working with the risks actively and continuously easy.
During our planning phase we identified two technologies that were critical to the design and implementation of our closed connected process: single-use (SU) components and sensors. To reduce the risk of contamination and maintain the quality of the product in a closed process, it is important to connect the unit operations using validated aseptic connectors. Aseptic connectors are available as single-use components in both connect and disconnect variants. Currently, an industry standard for connections is lacking and there are several distinct fittings available. It is therefore critical to map out each connection to make sure that various connections for each unit operation fit together. Ease of access as well as aseptic assurance to both the process stream and the sample should be considered when selecting aseptic connectors. Sampling locations and their significance to isolating an issue should be carefully thought out in advance as well.
Generally, if sensors are designed to monitor critical parameters, fewer samples are required in a closed process. Sensors can also further reduce the risk of contamination from on-line or in-line sampling. Various single-use capacitance sensors are available to monitor the viable cell density as well as disposable flow meters, load cells, pressure sensors, pH, and conductivity sensors. Flow sensors and weight scales were used in this study for tight control of shear rate, monitoring fresh media addition, as well as removal of spent media in the large-scale perfusion process. From our experience with the small-scale studies, we found that it would have been difficult to impossible to run a successful continuous large-scale perfusion process without such automatic monitoring.
Conclusion
Biomanufacturers strive to constantly improve manufacturing methods, costs, and timelines while ensuring product quality. Evaluation of new technologies and methodologies can be challenging. In this study, we challenged many of the newer trends in manufacturing including perfusion cell culture, fiber-based chromatography, and single, use closed connected processing.
We have shown that it is possible to successfully execute a closed connected process in a non-classified area. The closed set-up using ReadyToProcess disposable solutions made this possible, and the low bioburden and endotoxin levels confirm the success of our process setup. To run a process based on disposable solutions requires good logistics of all consumables day-by-day, which needs to be properly planned out prior to execution. Another point that is often de-prioritized is the arrangement of all buffers by process step to avoid crossing tubing and to minimize the footprint for buffer vessels. Last, but not least, communication is crucial to execute a process which requires cross-disciplinary teams and complex processes. By utilizing an agile way of working the communication and identification of team inter-dependencies were facilitated and under control.
There are many advantages to using a closed connected process instead of a traditional, disconnected biomanufacturing process. These advantages include shorter processing time, fewer manual interactions during the process, decreased CAPEX, reduced resources for cleaning and validation, and a smaller manufacturing space footprint. In our case study, we have shown that with proper planning, implementation and technology, it is possible to develop a large-scale continuous process that can yield a high-quality product, while meeting or exceeding manufacturing requirements.
Watch the digital series, Connecting the Dots, focused on a connected closed mAb process.